Let’s be real for a sec—“programmed cell death” sounds like something out of a sci-fi horror movie. But here’s the twist: your cells need this process to stay healthy. Think of it as your body’s built-in cleaning squad. Without it, you wouldn’t heal a skinned knee, grow strong muscles, or even develop into a human with separate fingers instead of a giant mitt. Yeah, that weird thing where embryos lose the webbing between digits? Totally orchestrated by PCD. Pretty wild, right?
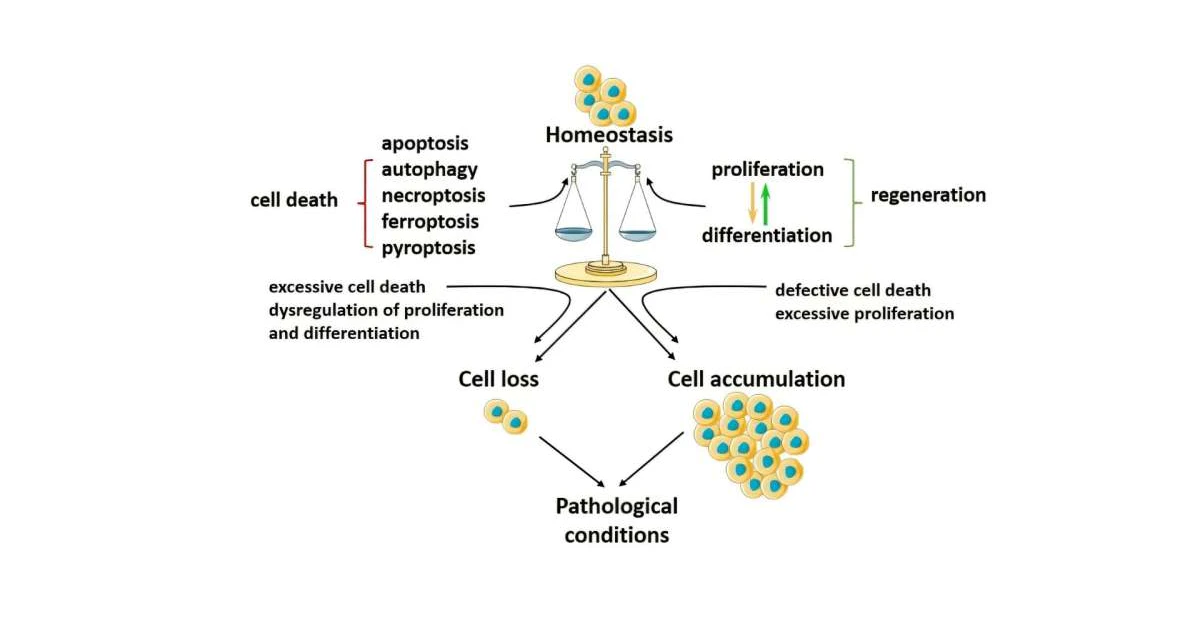
But here’s the kicker. When PCD gets stuck on “delete” or turns into a lazy control freak, things get messy. You end up with cancer when rogue cells won’t die, or neurodegenerative chaos like Alzheimer’s when too many neurons self-destruct. The balance is everything—like a Goldilocks situation. Too much? Not good. Too little? Also not good.
PCD: The Bodyguard That Occasionally Goes Rogue
Let’s break it down. Programmed cell death (PCD) isn’t some evil scheme. It’s a survival tactic. Your cells have a set of instructions, and sometimes those instructions say, “Yo, it’s time to check out.” Just like shedding dead skin cells, PCD keeps overgrowth, errors, and infections under control.
You know how your phone’s operating system deletes corrupted apps? PCD does the same for your cells. It’s clean, quiet, and efficient. For example, during limb development, cells in your hands and feet sign off so you don’t end up sculpted like a sausage—not ideal, right? According to Wikipedia, this exact process shapes your fingers and toes before you’re even born.
What Triggers a Cell’s Self-Destruct Button?
PCD isn’t random. It’s a response to specific cues—like your body whispering, “You’re not serving the team today.” These signals can come from DNA damage, infections, or even a neighbor cell tapping out due to overcrowding.
Take apoptosis—the polite way to die. Cells shrivel up, shrink their nuclei, and get quietly whisked away by cleanup crews like phagocytes. No drama, just efficiency. But flip the switch to chaotic PCD (we’ll call it “necroptosis” in nerd terms), and suddenly the body releases danger alarms, sparking inflammation. It’s like swapping a silent office cleanup for a full-blown warehouse fire drill.
Behind the Scenes: Autophagy’s Unique Job
Sometimes PCD isn’t an instant off-button. Autophagy is more like cellular extreme couponing. Cells recycle damaged parts—think broken mitochondria or junk DNA—to survive stress. It’s a key player in tissue regeneration, especially when you’re under the weather or recovering from injury. But here’s the catch: if autophagy goes berserk, it can weaken cells instead of strengthening them. Balance, again, is everything.
PCD’s Good Deeds: Tissue Revival and Beyond
Imagine your body as a construction site. Injuries happen. Cells get damaged. Enter PCD, tearing down unstable structures so repairs can begin. Without it, healing would stall. Dead cells would pile up like construction waste nobody bothered to clean.
Your Gut’s Hero: Apoptosis on the Clock
Did you know your gut lining renews itself every couple of days? The Molecular Biology of the Cell says your intestinal cells live fast, die young, and get replaced by their fresh, off-the-production-line cousins. PCD clocks this entire cycle. It’s why your digestive system stays resilient. Even after a questionable street taco binge.
When Injuries Turn Into Healing—PCD at Its Best
Take a scrape on your knee. Within hours, your body’s cell renewal kicks in. Damaged cells self-remove. Their clearance makes space for fresh tissue regeneration. It’s like the body knowing when to swap out burned-out light bulbs for new ones. If this cleanup fails? Scarring or infection sets in. Grim.
Case Study: Tadpoles and Their Disappearing Act
Back in school, you probably learned how tadpoles turn into frogs. Their tails vanish in the process. How? Through PCD. A 2005 NCBI review explains that swapping larval features for adult ones requires targeted cellular suicide. Without it, your frog would waddle around with a tail forever. Evolution’s kinder than that.
PCD Gone Wrong: Cancer, Fibrosis, and Neurodegenerative Diseases
Now, let’s talk about the downside. The same process that keeps your skin soft and your gut functional can also turn into a traffic jam when it misfires. Too much. Too little. Wrong place. Wrong time. Here’s what we’re up against:
Fibrosis: When the Cleanup Takes a Nap
Fibrosis that makes lungs or liver stiff? It’s often PCD’s fault—or lack of it. Diseased tissues build up scar tissue when cells won’t die like they should. Instead of clearing damaged fibroblasts, your body lets them hang around and go rogue. The lungs or liver suffer. And so do most patients with conditions like cystic fibrosis or cirrhosis.
Neurodegenerative Diseases: The Cell Death Spiral
Alzheimer’s, Parkinson’s… these conditions accelerate when neurons commit apoptosis faster than replacements can rer.
Table: Neurodegeneration vs. Cell Death Modes
| Disease | PCD Type Involved | Impact |
|---|---|---|
| Alzheimer’s | Excess apoptosis + ferroptosis (iron-driven death) | Neuron loss = memory fade + cognitive decline |
| Parkinson’s | Mitochondria-induced apoptosis | Loss of movement control |
| Multiple Sclerosis | Necroptosis (inflammatory death) | Myelin sheath degradation makes neurons stutter |
Are We Ready to Hack PCD Therapies? Curing Diseases One Gene at a Time
Cutting-edge treatments are starting to throw PCD a lifeline—or rein it in when it’s attacking the wrong targets. Venetoclax, approved for leukemia, forces cancer cells back into apoptosis’s good graces. It hacks BCL2, the protein that cancer uses as a “do not die” shield. And it works. Neat, right?
Targeting BCL2: How Venetoclax Gamed Cancer
Here’s the deal. In blood cancers, malignant cells shut off their death program to survive. Venetoclax flips that switch by binding to BCL2. According to PMC’s 2024 review, it’s reshaping what we thought was possible. But it’s not a free lunch. Sometimes, overstimulation = side effects. Like when targeting brain neurons overrides their regeneration pathway. Suddenly, diplomas of dead cells pile up faster than you can say neurodegeneration.
Could Neurodegenerative Diseases Retrain the Death Program?
On the flip side, researchers are nudging PCD pathways to slow down. Take Huntington’s disease—where excess apoptosis triggers neuron melt-down. Gene therapy and ferroptosis inhibitors (Nature’s 2024 article) are exploring whether throttling PCD could give these diseases the middle finger. But tread carefully—it’s still early days.
Why PCD Manipulation Is Easier Said Than Done
Scientific challenges? Plenty. For instance, PCD type detection often looks like a jumbled mess under the microscope. Apoptosis, necroptosis—they can blend together, making lab results tough to call (PMC’s detection problem). And if detection’s dodgy, how do we tweak treatments? That’s the million-dollar question.
Spotting the Problem Before It’s Too Late: PCD Detection Methods
So here’s the nerdy part. How do scientists know if PCD’s misbehaving? It’s not like cells send a text: “Hey, I’m about to die.” They use clever tricks instead.
Old Faithful: The TUNEL Assay (for DNA damage!)
The TUNEL assay clocks DNA fragmentation, a major apoptosis tell. If a cell’s genome is literally chopped up? Drama queen’s got issues. Back in PubMed’s 2022 review, they even compared TUNEL to a DNA death certificate. Solid start, but misses the full PCD family—not just apoptosis. Necrosis, pyroptosis, and ferroptosis leave different fingerprints.
New School: AI-Driven PCD Trackers
Folks are now teaching AIs how to read what cell death “feels like.” (Reactome’s data schema team) is categorizing PCD types based on hyperspecific biomarkers. The goal? Let machines spot a cell’s demise in real-time—like giving scientists X-ray vision. But here’s the kicker: these systems are still in the lab. So don’t expect an app version—yet.
It’s Not the End of the Story… Just the Beginning
Let’s circle this back. PCD is the reason you got to read this article: too much death kept neurons alive during your learning phase; too little, and your body would’ve gone awry long ago. It’s a double-edged sword, sure, but one we’re learning to wield.
The rest isn’t about villainizing PCD. It’s about understanding the mechanism. Ask any researcher, and they’ll emphasize one thing: we’re barely through the door. New therapies, cleaner assays, and smarter systems are on the horizon. Much of it hinges on that simple truth—the balance is everything.
So what now? Keep an eye out for advances. Or better yet, let your curiosity lead you deeper. If this got your brain bubbling, drop a question. We’re here to decode the sciencey stuff—no jargon, no fluff. Just plain talk about cellular life, death, and the line between genius and disaster.
Programmed cell death isn’t a script for tragedy. It’s a playbook for balance. And the better we understand it, the better we’ll perform when things go off track.




















Leave a Reply
You must be logged in to post a comment.