When you hear the words “cancer treatment outcomes,” you might picture a jumble of statistics and medical jargon. In reality, they’re simple ideas that tell us how long people live, how well they feel, and what the road ahead might look like after therapy. Knowing these outcomes helps you ask the right questions, weigh the benefits and risks, and feel more in control of your journey.
Below, I’ll walk you through the most important pieces of the puzzle—survival numbers, quality‑of‑life scores, the role of new technologies, and even the hidden disparities that affect many patients. Think of this as a friendly conversation over coffee, where I share facts, stories, and a few tips that could make a real difference for you or a loved one.
Defining Outcomes
What “Outcome” Really Means
In oncology, an “outcome” is any measurable result of treatment. The three most common ones are:
- Overall Survival (OS) – the length of time from diagnosis (or start of treatment) until death from any cause.
- Progression‑Free Survival (PFS) or Disease‑Free Survival (DFS) – the period during which the cancer does not grow or return.
- Health‑Related Quality of Life (HRQoL) – how patients feel physically, emotionally, and socially while living with or after cancer.
All three matter. A therapy that adds months to life but leaves you bedridden isn’t a win for most people. Conversely, a treatment that improves comfort but shortens life dramatically can feel like a false promise.
How Doctors Track These Numbers
Outcomes are recorded in massive databases such as the SEER program and the National Cancer Database (NCDB). Researchers also use patient‑reported outcome measures (PROMs) to capture the day‑to‑day experience of living with cancer. For example, a recent NCDB study of 70,876 stage II–III colon‑cancer patients showed that patients who received chemotherapy lived, on average, 3 years longer than those who did not—a clear illustration of how real‑world data shape our understanding of outcomes.
Typical Survival Numbers (2020‑2024)
| Cancer Type | 5‑Year OS (Stage II‑III) | Median PFS (months) |
|---|---|---|
| Colon | 68 % | 48 |
| Breast (HER2‑negative) | 85 % | 78 |
| Non‑small‑cell Lung | 31 % | 22 |
Key Shaping Factors
Tumor‑Related Factors
The stage at diagnosis, the exact histology, and molecular fingerprints (like KRAS, EGFR, or HER2 status) all drive outcomes. Early‑stage disease usually means better survival, while aggressive subtypes can be stubborn even with the best therapies.
Patient‑Related Factors
Age, other health conditions, and where you live matter a lot. A Nature study on racial and socioeconomic disparities found that Black patients still lag behind White patients in survival for many cancers, even after adjusting for income and stage. This isn’t just a number—it’s a reminder that access to high‑quality care, clinical‑trial enrollment, and supportive services can tilt the odds.
Treatment‑Related Factors
The choice between surgery, radiation, chemotherapy, targeted therapy, or immune checkpoint inhibitors can swing outcomes dramatically. For many solid tumors, combining modalities (for example, surgery + adjuvant chemotherapy) has become the gold standard.
Case Study: Neoadjuvant Therapy in Esophageal Cancer
When doctors give chemotherapy before surgery (neoadjuvant), they often shrink the tumor, making removal easier and improving survival. Yet some patients develop Chemotherapy effectiveness prediction challenges—known as neoadjuvant chemotherapy resistance. Understanding why a tumor resists treatment can help clinicians adjust the plan before it’s too late.
Chemotherapy Effectiveness
Why Predicting Response Matters
Everyone wants a treatment that works—without the nasty side‑effects. Predictive tools using genomics or AI‑driven models aim to tell us, before the first infusion, whether a tumor will respond. When predictions are accurate, patients avoid unnecessary toxicity and doctors can pivot to alternative strategies.
Current Predictive Tools
Many cancer centers now run tumor‑sequencing panels that feed into a Precision oncology platform. These platforms compare your tumor’s genetic makeup to thousands of previous cases, providing a probability of response to each drug class.
Mini‑Guide: Reading a Molecular Report
- Identify the driver mutations (e.g., KRAS, BRAF).
- Look for actionable alterations—genes with approved targeted drugs.
- Check the “tumor mutational burden” – high scores may predict benefit from immunotherapy.
- Ask your oncologist how these findings translate to your specific treatment plan.
Esophageal Adenocarcinoma
Standard of Care Today
For most patients, the current standard blends surgery with neoadjuvant chemoradiation. This approach improves 5‑year survival to roughly 45 %, a big jump from the 20‑30 % seen with surgery alone a decade ago.
Resistance in the Real World
Unfortunately, not every tumor shrinks. Esophageal adenocarcinoma treatment experts are studying why some cancers become resistant to the pre‑surgical regimen. Factors include tumor hypoxia (low oxygen) and specific gene mutations that deactivate chemotherapy. When resistance is identified early, clinicians may add a different drug or move directly to surgery, preserving the chance for cure.
Story From the Clinic
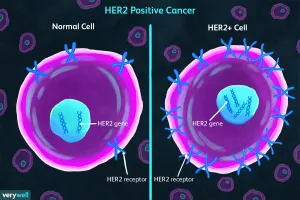
John, 58, was diagnosed with stage III esophageal adenocarcinoma. After three cycles of neoadjuvant therapy, his scans showed the tumor barely moved. His team performed a molecular test that uncovered a HER2 amplification. Switching to a HER2‑targeted drug turned the tide—post‑surgery pathology revealed only microscopic disease. John’s experience illustrates how personalized testing can convert a “dead‑end” into a hopeful path.
Outcome Disparities
Who Is Falling Behind?
Data consistently show that Black, Hispanic, and low‑income patients experience lower survival rates across many cancers. The reasons are complex: fewer high‑volume treatment centers in their neighborhoods, delayed diagnoses, and under‑representation in clinical trials.
Root Causes
Limited insurance coverage often means patients can’t travel to top‑rated facilities. Additionally, cultural mistrust of the medical system can deter trial participation, which is a key driver of progress.
What You Can Do
- Ask your oncologist about the hospital’s volume for your specific cancer—high‑volume centers usually have better outcomes.
- Search for clinical trials that are open to diverse participants.
- Lean on patient‑navigator programs; many cancer centers offer free assistance to help schedule appointments and manage transportation.
Side‑Effect Balance
Common Dermatologic Toxicities
Skin reactions are perhaps the most visible side‑effects of modern therapies. Rashes, hand‑foot syndrome, and pigment changes can be uncomfortable and sometimes lead patients to stop treatment early.
How Side‑Effects Influence Outcomes
When side‑effects are severe, adherence drops, and the benefits of a regimen shrink. Managing skin health proactively—using moisturizers, protecting skin from sun, and early dermatologist referral—keeps you on track.
Quick‑Tip List
- Apply a fragrance‑free moisturizer after each shower.
- Wear loose, breathable clothing during chemotherapy.
- Use sunscreen with SPF 30+ even on cloudy days.
- Report any new rash to your care team right away.
Future Trends
AI‑Driven Prediction
Artificial intelligence is training on millions of cancer records to forecast outcomes with uncanny accuracy. Early pilot studies suggest AI can identify patients who will benefit most from combination immunotherapy plus chemotherapy, shaving years off the time to disease progression.
Combination Immunotherapy & Chemotherapy
Government “Moonshot” initiatives have accelerated trials that pair checkpoint inhibitors with standard chemo. Results from 2023‑2024 show a 7‑point rise in 5‑year survival for metastatic lung cancer—an encouraging sign that the future may bring more “win‑win” scenarios.
“Moonshot” Impact
By funneling billions into research, the United States has seen a steady rise in cancer survival over the past two decades, even while other leading causes of death lag behind. Continued investment means more rapid translation of lab discoveries into bedside care.
Decision Roadmap
Ask Your Team
Before you sign any consent form, consider these questions:
- What is my estimated overall survival with each option?
- How will my quality of life be affected in the short and long term?
- Are there biomarkers that can guide therapy selection?
- What support services (nutrition, mental health, financial counseling) are available?
Personal Checklist
- Stage & Molecular Profile: Confirm you have the latest staging scans and a comprehensive genomic report.
- Treatment Preference: Write down what matters most to you—longevity, side‑effect tolerance, time off work, etc.
- Support Network: Identify a friend or family member who can accompany you to appointments.
- Financial Planning: Check insurance coverage, explore patient‑assistance programs, and consider a financial counselor.
Having this roadmap in hand turns uncertainty into action steps you can control.
Conclusion
Cancer treatment outcomes are more than numbers on a chart; they’re a blend of survival time, how you feel day‑to‑day, and the personal values that shape your decisions. By understanding the factors that drive outcomes—tumor biology, patient health, treatment choice, and even social determinants—you empower yourself to have informed, honest conversations with your care team.
Remember, the best outcomes start with the best information. Download the printable decision‑making checklist, talk openly with your oncologist about predictive testing, and don’t hesitate to seek high‑volume centers or clinical‑trial options if they fit your goals. You deserve a treatment plan that respects both your longevity and your quality of life.
What has your experience been with cancer outcomes? Have you found a tool or a doctor who helped you navigate these choices? Share your thoughts, and let’s keep the conversation going.














Leave a Reply
You must be logged in to post a comment.