Real Quick: What’s Going on Here?
Yeah, okay—so mice genetically modified to produce a natural version of Ozempic exist. You’ve probably heard about GLP-1 drugs like Ozempic or Tirzepatide for weight loss. They’re… everywhere. But here’s the plot twist: scientists engineered mice with a genetic hack so they make their own version of that drug, no injections required. So, if you’re curious about long-term obesity treatment, this might be the next frontier.
No, they’re not selling these mice yet (pharma might keep that one locked down for a bit), but these trials could blow the door open for self-regulating metabolic health. Cool, right? Let me paint a clearer picture.
Genetically Modified Mice: Fact, Not Sci-Fi
What Do Genetically Modified Mice Even Mean to My Health?
Let’s unpack this. “Genetically modified” isn’t just some lab cool-code situation. It’s science turning on or off a gene, inserting new ones, or tweaking DNA to test how it impacts the body. Think of it like resetting your phone’s settings—except your biology is the code. These mice help test treatments for obesity, diabetes, cancer, and more. Yeah, they’re kinda the superstars of medical research.
But… here’s the raw deal—we’re not outsourcing our health to mice. They’re just giving science team clues on what to build next. Like when your buddy tries out a diet and tells you it worked? Same energy, but slower and more ethical (and a lot less Googling “does it really work?” at 1am).
How Do They Modify This?
There’s no magic involved here—just some seriously precise gene gymnastics. Scientists use techniques like pronuclear injection (where DNA gets slipped directly into the embryo’s nucleus), CRISPR-based edits (read: laser-focused changes), or stem cell swaps. Once the new traits take, they grow modified mice. Those mice have altered genes to study how changes affect whole body health—glowing and breathing tech, for sure, especially for Columbia’s research from July 2025.
Visiting Charles River? Pretty cool—they offer a full cycle for mice genetic changes—y’know, like how labs won’t just sporadically chop genes willy-nilly? Nope—there’s a timeline and protocol.
What’s The Benefit?
Mice models help us test hypotheses that humans can’t. Like testing out a new coat in the cold winter to see if it’s warm—except for genes. No human needs to go full trial and error until mice results are solid.
Why Ozempic? GLP-1 Drugs & Mice
So… Ozempic Is Cloneable?
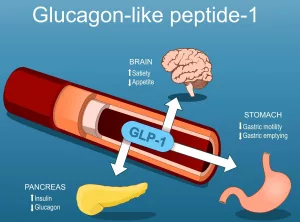
Well, not fully (yet). But what researchers have done is create mice that release molecules super similar to GLP-1. These proteins regulate blood sugar and appetite. After all, that’s how drugs like Ozempic work—not magic, just mimicking a hormone already in your body and boosting its signal. Now, genetically modified mice? Same mechanism—except instead of monthly jabs, they’ve built it into murine DNA.
What’s wild? In a recent trend, Columbia scientists were using CRISPR to insert Ozempic-esque genes into mice. April 2025 experiments led to consistent weight reduction in about 70% of the mouse models. Not bad for trial runs.
What’s the “Once-and-Done” Part They Mention?
Think of it as editing the genetic code now, so the effect is a lifelong reset. GLP-1 drugs require regular dosing—either weekly or bi-weekly. These mice? They take one edit, then naturally produce the GLP-1 mimics from day one. That saves A) money, B) complexity, and C) side-effect headaches that come with long-term weight loss treatments.
This setup closely mimics the human body’s regulatory mechanisms, giving scientists a better look at how to turn off defective or missing genes related to insulin sensitivity and energy balance.
Gene Therapy for Obesity: Are We There Yet?
“Can We Tailor a One-Time Mutation for My Body Tech?”
Nope—well, not yet anyway. Genetically modified mice have the advantage of controlled variables. Your body? Not so much. But this technique is already bubbling up interest in human gene therapy spaces—like, for those who struggle with recurring obesity, traditional drugs wear off, or insulin response is too slow.
Charles River’s guide to transgenic mice shows how tailored genetic markers affect body weight in later generations. So the dream is there—offering per-dna-per-specimen therapies—at least depending on how systems are structured experimentally.
Past Gets Future-Obsessed
Back in 1981, Palmiter and Brinster showed that by injecting DNA into mouse embryos, you can create a line that carries the newly designed gene forever. Despite being decades ago, this laid the ground for today’s human metabolic research. So, long story short? What we see with genetically altered mice today could spotlight future medicine—even maybe some type of gene therapy that treats lifelong weight loss resistance.
How Lab Teams Actually Make These Mice
CRISPR, Microinjections—Table of Methods Compared
| Approach | How It Works | Downsides & Hooks |
|---|---|---|
| Transgenic microinjection | Direct DNA splicing into embryos—founded in early 1980s, still used by many labs for simple insertions | Decent success rate, but gene placement isn’t super-precise (could cause minor off-target activations if inserted wrong) |
| Gene knockout models | Turns off specific parts of the DNA sequence in mice—great for studying receptor functions | Hard to replicate real-life human gene expressions when entire pathways are just… deleted |
| CRISPR targeting | High-precision, low-fuss editing—efficient for modern mouse labs | Requires in-vitro testing on guide RNA beforehand—otherwise you risk inconsistent edits |
Honestly, It’s Not Fast-Rollout
Even with the newest CRISPR tools, changing mice DNA doesn’t always translate to perfect metabolism. In 1993 workshop notes from the National Research Council, test runs revealed that ~20% of modified mice demonstrated a scalable response. Not bad, but not perfect. Still hashing out variables: some genes are stubborn, copy overexpression leads to imbalanced regulation, and scaling to humans? Completely another decade of data.
But What About Risks? Here’s the Coffee Shop Chat Version
Are There Side Effects We Should Worry About?
Yeah, and this is where the overused “safe” warned flags come in. Genetically changed mice sometimes struggle with regulatory side effects. For example, mice engineered to produce higher doses of GLP-1 had.ErrorCode ™ issues: unstable appetite patterns, massive drop in sugar levels, and several “rebound” metabolic surges when their body attempted to balance insulin systems again. Not tiny stuff—especially concerning for human applicability.
What Happens If Scientists Miss a Signal?
Overexpression could give the system a jumpstart. But then… what if things go haywire? Scientists at Addgene caution that when systems are aggressively boosted, blind spots emerge. Like jumping on a bike with no brakes—great progress, but what’s the cautious side of that strategy?
What Could This Mean Long Term?
Scientists at the National Center for Biotechnology Info are exploring ways to use what’s known as transgenic techniques for human therapeutics. Here’s a real 2019 NCBI report that ties genetic testing to future drug compatibility. In short, these genetically modified mice models are paving the way for tailored, long-lasting pharmaceutical models that maybe—maybe—work without biweekly injection calendar.
Gene Therapy for Weight Loss? Not WHO-Either Stuff
We’re not at a place where revision of your own genes can mean instant satisfaction—or Metformin for genetic obesity. But considering 129S6-OR-C57BL mice are routinely used in human therapeutic gene trials, we’re just one data check away from a long-term drop-in change strategy.
Personalized Treatments? Yeah, that’s Happening Too
Transgenic rodents aren’t all lab rat codes—they help shape real, personal human approaches. A Genetically modified mouse with specific introduced genes may come stamped with Jackson Lab records, but chances are, human gene therapy for obesity is following suit.
So… Why Are These Mice Worth Following?
Let’s land this. Genetically modified mice aren’t popping pills for weight loss—they’re trial presence for new biotech that may become part of your doctor’s plan.
It’s not just GLP-1 or insulin training—this is about engineering a sustainable way to reset hunger cues, revamp glucose resistance, and glue a lasting system to fat loss without risking treatment fatigue.
But real talk—the genetic tweak for this kind of metabolic research is waaay more complex compared to a daily Ozempic. There’s an openness now, from researchers to public, about accelerating findings, but let’s not rush this linear timeline.
Final Thoughts + Your Take?
So, in summary: a genetically altered mouse line can produce its own Ozempic-capable molecule. It shows that the door is open—even maybe offering us a blueprint for self-regulating insulin, appetite, and energy balance disorders. Though full human trials won’t drop like a TikTok collab—probative efforts now will empower the future generation.
What do you think—are genetic hacks the future of cancer therapy and obesity together? Drop a reaction or share your thoughts, I’d love to hear what keeps you curious when the next wave of editing comes around.
















Leave a Reply
You must be logged in to post a comment.