Did you ever wonder why humans get cancer way more often than our closest animal relatives, like chimps? It’s not just about stress or our lousy fast-food habits.
A team of researchers at UC Davis discovered that losing a single genetic mutation might be why our immune cells can’t fight solid tumors as well as chimps’. Yeah, you read that right—cells. And it’s tied to a small but sneaky biological trade-off we made as a species over millions of years.
More Than Just “Bad Luck”
Listen, I get it. Cancer feels random. Scary. Unfair. But what if I told you that some of our genetic vulnerabilities actually have a messy, complicated origin story? One we didn’t choose, but our ancestors did? Sort of.
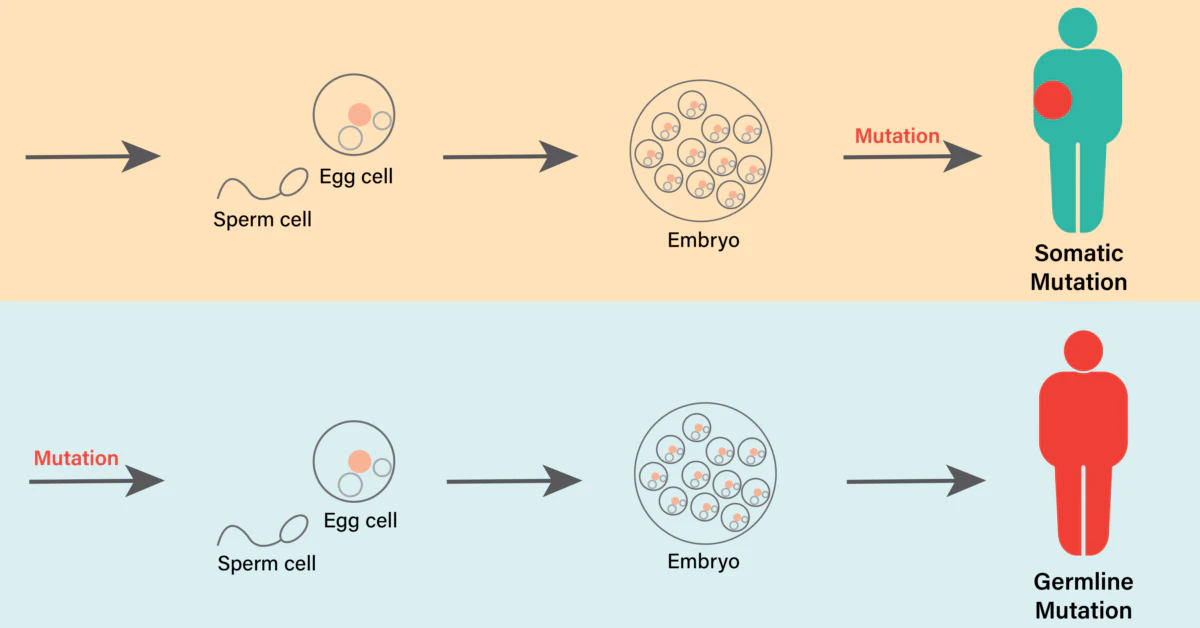
Let me paint you a picture: Humans share around 98.8% of their DNA with chimps. But somewhere along the way, we lost a specific sugar molecule called Neu5Gc. This gene (CMAH) stopped working. Basic gene change, right? Except, here’s the kicker—this mutation may also have made our immune cells less effective at detecting and killing solid tumors.
Chimp Genes: More Cancer-Resistant Than Ours?
You might think chimps are “just animals”—but their immune systems are doing things ours don’t anymore. How? Something about Neu5Gc, the missing sugar molecule in humans. Chimp immune cells recognize bodies that have this sugar as foreign, which means they attack hard when tumors start to grow. Our system? Not so much.
So, this isn’t just a random “we got unlucky” situation. It’s a genetic mutation with consequences. And some of those consequences might now be fueling breakthroughs in solid tumor treatment.
Why Do Human Immune Cells Suck at Fighting Solid Tumors?
Think of your immune system like a team of cops scanning traffic for bad behavior. Chimp cops? They’ve got a license plate to look for—Neu5Gc. They pull over anything suspicious. Fast. Efficient.
But humans? All traces of Neu5Gc cooked out of our systems due to the CMAH mutation. Now, our cops can’t tell which cars are bugs or which are innocent bystanders. They let suspicious vehicles (tumor cells) keep going, unnoticed.
Some researchers are now asking: Can we train our immune cells to “see” those missing tags again? A recent Dana-Farber study suggests that tweaking immune signaling pathways might help our cells start pulling over tumor cells like our chimp cousins’ do.
Humans vs. Chimps: A Molecular Tug-of-War
Chimps, retaining their Neu5Gc, have an edge when it comes to solid tumor defense. Humans, with a weakened immune response, have to rely on other tools—like early detection and treatment—to pick up the slack.
| Factor | Humans | Chimps |
|---|---|---|
| Neu5Gc Production | Mutated (inactive CMAH gene) | Natural Neu5Gc production |
| Early Cancer Detection | Come on, doc… not until it’s full-blown | Acts fast. Immune system on high alert |
| Treatment Research Implications | Fighting to “reprogram” immune cells | Source of inspiration… look at their immunity |
What Does This Mean for Human Cancer Research?
In short? Scientists aren’t just looking at cancer drugs anymore. Now, they’re looking at evolutionary cancer research to build brand-new solid tumor treatment strategies.
Here’s the real messy part—genes like CMAH don’t exist in a vacuum. They’re part of a network. Tweak one thing, and other functions might change too. It’s not a rewind-to-chimp button. It’s more like a DNA remix session—one that has to avoid boosts in inflammation and other risks.
Not All Mutations Are Created Equal
Let’s take a shift here. Not every cancer genetic mutation makes headlines.
Many occur in unrelated sections of DNA. Sometimes, junk mutations don’t even affect gene expression. But some—like the tumor suppressor gene TP53—have a more direct line to cancer development.
Inherited vs. Acquired Mutations
You can get these mutations two ways—either at birth or later, from your lifestyle or just plain DNA-copying screw-ups. Inherited ones? Only about 5–10% of all cancers are tied to these “built-in” gene faults.
Acquired mutations? Those come from random changes in genes as your DNA replicates, say, in a sunburnt cell or a stomach cell exposed to smoke. Not glamorous, but common. According to the National Cancer Institute, most cancer mutations fall into this messy, unromantic category.
5 Common Hereditary Cancer Mutations
- BRCA1/2 – Breast, ovarian, prostate
- APC – Colorectal cancer
- MLH1/MSH2 – Lynch syndrome (ulcerative, huh?)
- PTEN – Syndromes like Cowden’s
- TP53 – Li-Fraumeni (pediatric cancers, bone tumors)
Gene Mutations in Real Life: Stories That Matter
I once met a woman named Anna at a survivorship fundraiser. Her mother had ovarian cancer in her 50s. Anna found her own carcinogens in a genetic test—BRCA1 mutation. She had prophylactic surgery. Took action. You better believe her story isn’t unique.
Mutations can’t scream “I’m here!” They lie dormant for years. Some have built-in early warning systems. Some don’t become clear until things feel too far gone. That’s why genetic mutation isn’t “just science.” It’s prevention. It’s risk. It’s action. It’s personal.
What Are the Red Flags for Inherited Mutations?
Time for a buddy check-in.
- Cancer before age 50
- Multiple primary cancers
- Same cancer across more than one blood relative
- Ancestry tied to known cancer-causing genes (e.g., Ashkenazi Jewish, African American BRCA2)
- Cancers “uncommon” in certain groups (like male breast cancer)
If your family has three or more of these, you’re both living a mystery novel and in a place where medical-grade genetic testing would shut up really useful questions. You can’t move forward in confusion.
So, What Can Gene-Driven oncology actually DO for Us?
It’s not all doom and mutation doomscrolling. The study of genetic mutation is opening new paths in solid tumor treatment. You’ve probably heard of drugs that target specific cancer-linked genes—like PARP inhibitors for BRCA mutations, or EGFR blockers for lung cancer.
What’s cool is that now, some of this research isn’t just targeting cancer cells—they’re targeting immune cells, trying to make them work like our chimp relatives. Some trials include glycans, the “sugar coat” our cells wear. They’re a bit like trick-costuming your immune system. Butch Cassidy and the Sundance Kid, immune edition.
Can You Treat Cancer Based on Your Genes?
Short answer? Yes.
Long answer: gene mutations act as roadmaps. If you have a mutation (+ therapy), you can sometimes find really targeted medicine. BRCA2? Ovarian and breast cancer? There’s a PARP pill for that. And now, some researchers think altering “missing” glycan signals might help your body see tumors like a chimp’s does.
Examples of Genetic Mutations and Their Targeted Treatments
| Mutation | Treatment | Why It Works |
|---|---|---|
| BRCA1/2 | PARP inhibitors | They target the weaknesses in DNA repair in tumor cells |
| EGFR (Lung) | Osimertinib | Acts like a GPS to disrupt faulty protein signaling |
| Lynch syndrome genes | Immunotherapy helpers (e.g., pembrolizumab) | Boost your immune system when tumors evade detection |
Stopping Mutations Before They Happen… Is That Possible?
Let’s get real—it’s not like you can just stop DNA from copying itself wrong. Our cells divide. Mistakes happen. But what if you could reduce chances of harmful mutations?
Feel like you’re out in the sun too much? Maybe invest in a hat. More interested in mutation tracking than hats? Ask your doc about tumor genomic testing. Figure out what’s present, not just what’s inherited.
If you have a family history, diet and stress management might shift cancer risk. Research says certain mutations involve inflammation and oxidative stress—can you control that? Somewhat. Not fully, but partially.
Testing for Genetic Mutation: Not Just a Theory
So, how do we even know we have inherited gene faults or not? Two main paths:
- Germline Genetic Testing – checks if you’re born with a mutation. Think BRCA1 cheating-coded from your mom or dad.
- Tumor Genomic Testing – looks after cancer forms for specific mutations the tumor made. These tell docs what drugs might work—but they aren’t passed down.
They both matter. But only one impacts your kids. A Dana-Farber article explains, “Understanding this helps personal treatment plans.” Would you want your oncologist guessing? I didn’t think so.
Why Even Bother with “Harmful” Mutations?
This might sound weird: Not every harmful mutation is all that harmful. Some are like car alarms with a short fuse. They go off too much, in the wrong context. Others are like a sleepy break-in – cancer’s happening, but mutation not driving it big time.
Same with “passenger mutations” in cancer. They’re along for the ride. Not controlling cancer growth, just existing. The tricky part? Separating driver from passenger mutations takes time, data, and focus.
Can Your Body Fix Genetic Mutations Automatically?
Here’s the positive: Your DNA repair systems are okay at their job. Most mutations don’t result in cancer—because cells have “self-destruct” mechanisms. MMR (mismatch repair) checks DNA as it copies. If it’s messed up? It self-corrects or flags it.
But what if the mutation isn’t detected? That’s when you get tumor growth. That’s also when oncologists start needing a detailed mutational profile to find targeted treatment.
What Do Passenger Mutations Even Mean?
So. You found a mutation in a tumor. Cool. Are you sure it caused cancer? Are you seeing if this mutation also exists in healthy people? There’s this big database of mutations called COSMIC. It helps interpret which ones are solid tumor drivers and which are just… extra DNA fluff.
Oncologists and researchers aren’t just randomly applying mutations anymore. They’re diagnosing—reading patterns like we finally understand what these changes mean for a specific cancer.
Empowering New Insights from Old DNA
Fact: We’re not chimps. We’re not trying to be. Well, not seriously. But we can learn from the genetic mutation that evolved in their lineage—and ours—in a way that now informs cancer treatment advances.
Maybe gene change isn’t about fighting cancer. Maybe it’s about playing smarter with what we’ve got. Learn why that one mutation happened, why it impacted our immune system, and how to counteract it with a little nano-driven immunotherapy. Maybe genetic change doesn’t feel like a death sentence anymore. Maybe it feels like a clue—one that could lead to future treatment design.
Weaving evolutionary research with genetic clues, this study touches every part of oncology. Because if chimps can create better tumor defenses because of one gene—can we find a way to tweak our own systems? Possibly. Maybe even try.
Where Do You Go From Here?
You don’t have to Google “how to fix my DNA” and spiral into a panic. But understanding your family history? That matters. Feeling equipped to ask your doctor about tumor genomic testing? Even better.
If you’re under 50 and seeing cancer in your elders? Don’t just sit with it. You’ve got tools. If you already have cancer, mutation is a tool too—it helps doctors find viable treatment options based on tumor type.
Please keep one thing in mind: genetic mutation might explain risk, but it doesn’t equal fate. It gives us navigation. It helps us start asking harder questions. Because in cancer research, questions are just as important as answers. Sometimes more.
We can’t all be chimps. But we might be able to learn how to make our immune systems work smarter—and maybe stop those sneaky tiny tumors before they think they’ve won.
Could This Mutational Insight Change Your Approach to Cancer?
Tell us what you think. Your experience with genetic mutations or family history matters here. Share, comment, or stumble into our Chat box. Because none of us should go it alone when it comes to understanding cancer genetic mutation.















Leave a Reply
You must be logged in to post a comment.