Quick Answer Overview
In short, Monjuvi (tafasitamab‑cxix) is an FDA‑approved therapy that helps adults whose diffuse large B‑cell lymphoma (DLBCL) or follicular lymphoma has come back or didn’t respond to previous treatment. When paired with rituximab and lenalidomide, it can push progression‑free survival out to about 22 months—roughly cutting the risk of disease progression by more than half. The upside is compelling, but the drug also brings a handful of side‑effects that you’ll want to weigh carefully with your oncologist.
Think of Monjuvi as a new teammate in the fight against lymphoma, one that works hand‑in‑hand with existing medicines to give your immune system a stronger punch at the cancer cells. Below we’ll walk through the science, the data, the safety checklist, and what a typical treatment cycle looks like—so you can feel confident asking the right questions at your next appointment.
FDA Approval Update
Monjuvi’s story began in 2020 when the U.S. Food and Drug Administration (FDA) granted accelerated approval for relapsed or refractory DLBCL in patients who cannot undergo autologous stem‑cell transplant. The approval was based on overall response rates from the pivotal L‑MIND trial. Fast forward to June 18 2025, the agency expanded the label: Monjuvi now has accelerated approval for relapsed or refractory follicular lymphoma when given together with rituximab (Rituxan) + lenalidomide (Revlimid). This new indication was highlighted in the FDA’s press release that noted a median progression‑free survival of about 22 months and a >50 % reduction in disease‑progression risk.
Accelerated approval means the FDA allowed the drug onto the market because early signs looked promising, but further confirmatory trials are still required to verify long‑term benefit. According to the FDA label, the confirmatory study is already underway, which gives patients hope that the therapy may become a standard of care once full data are in.
For a deep dive into the regulatory timeline, check out our Monjuvi FDA approval article.
Mechanism Of Action
Monjuvi is a CD19‑directed cytolytic monoclonal antibody. In plain language, CD19 is a marker that lives on the surface of most B‑cell lymphomas. Monjuvi binds to this marker and flags the cancer cell for destruction. It does this in two major ways:
- Engaging the immune system: Once Monjuvi latches onto CD19, natural killer (NK) cells and other immune effectors recognize the antibody’s tail and launch a targeted attack, releasing toxic substances that kill the tumor cell.
- Direct cell death: The antibody itself can trigger internal signaling pathways that push the malignant B‑cell into apoptosis (programmed cell death).
Adding lenalidomide (Revlimid) amplifies this effect. Lenalidomide is an immunomodulatory drug that boosts NK‑cell activity and reduces the tumor’s ability to hide from the immune system. When you throw rituximab into the mix—a CD20‑targeted antibody—the three‑drug combo forms a “one‑two‑punch” against the lymphoma, hitting two different surface proteins (CD19 and CD20) while also rallying the immune response.
Clinical Efficacy Data
What really matters is whether these mechanisms translate into real‑world benefit. The L‑MIND trial, which enrolled 81 adults with relapsed or refractory DLBCL, reported an overall response rate (ORR) of 57 % and a complete response (CR) rate of 40 %. Median progression‑free survival (PFS) was 7.9 months, and the median overall survival (OS) has continued to climb as longer‑term follow‑up accumulates.
When the same drug combo was evaluated in relapsed/refractory follicular lymphoma (the 2025 extension study), results were even more striking. Patients who received Monjuvi + rituximab + lenalidomide experienced a median PFS of 22 months—over a year longer than many historical controls. The hazard ratio for disease progression was 0.48, meaning the risk was cut nearly in half.
To put those numbers in perspective, consider a hypothetical patient who would otherwise expect disease progression around the 10‑month mark; with Monjuvi, that timeline stretches to almost two years, offering valuable time for additional therapies, personal milestones, or simply more moments with loved ones.
For a side‑by‑side view of how Monjuvi stacks up against other relapsed lymphoma drugs, see the comparison table below.
| Drug | Approved Indication | Median PFS (months) | ORR (%) | Key Adverse Events |
|---|---|---|---|---|
| Monjuvi + Rituximab + Lenalidomide | Relapsed/Refractory Follicular Lymphoma | 22 | 62 | Neutropenia, Infusion reactions, Infections |
| Polatuzumab Vedotin + R‑CHP | Relapsed/Refractory DLBCL | 9.5 | 45 | Peripheral neuropathy, Cytopenias |
| CAR‑T Cell Therapy (axicabtagene) | Refractory DLBCL | ~12 (varies) | 82 | Cytokine release syndrome, Neurotoxicity |
For an overview of alternative therapies, our relapsed lymphoma drugs page provides deeper context.
Safety Profile Overview
Every medication has pros and cons, and Monjuvi is no exception. Below we break down the most common and the most serious side‑effects, plus practical tips to keep them in check.
Common (≥ 10 %)
- Fatigue or feeling “run down”
- Diarrhea
- Cough
- Fever (often a sign of infection)
- Swelling of the hands or lower legs
- Respiratory tract infections (e.g., bronchitis, sinusitis)
- Decreased appetite
Serious / Grade 3‑4 (≈ 30 % of patients)
- Infusion‑related reactions – fever, chills, flushing, shortness of breath; usually happen during the first two cycles. Premedication with acetaminophen, antihistamine, and a steroid helps prevent most of them.
- Myelosuppression – neutropenia (low neutrophils) in ~25 % of patients; thrombocytopenia (low platelets) in ~12 %; anemia in ~7 %. Regular CBC monitoring is essential.
- Infections – 30 % of patients experienced grade ≥ 3 infections; pneumonia was the most frequent serious infection (7 %). Prompt reporting of fevers (≥ 38 °C) and any signs of infection can lead to early intervention.
- Embryo‑fetal toxicity – both Monjuvi and lenalidomide can harm a developing fetus. Women of child‑bearing potential must use effective contraception during treatment and for at least three months after the final dose.
Management strategies are straightforward: pre‑medicate, keep a daily log of temperature and any new symptoms, and maintain close communication with your care team. For the nitty‑gritty on dosing adjustments and monitoring, see the “Treatment Journey” section.
Eligibility And Cautions
Monjuvi is indicated for adult patients (≥ 18 years) who meet one of the following criteria:
- Relapsed or refractory DLBCL that is not eligible for autologous stem‑cell transplant.
- Relapsed or refractory follicular lymphoma (after at least two prior lines of therapy) when given with rituximab + lenalidomide.
Patients should have adequate organ function (liver, kidney) and a baseline absolute neutrophil count ≥ 1,000 µL. Pregnancy is an absolute contraindication, and strong caution is advised for those with active uncontrolled infections or severe cardiac disease.
If you’ve been labeled “refractory”—meaning your lymphoma didn’t respond to prior regimens—our refractory lymphoma therapy guide outlines why Monjuvi can be a viable option when other treatments have fallen short.
Treatment Journey Steps
Understanding the schedule can demystify a lot of anxiety. Here’s a step‑by‑step look at a typical Monjuvi course.
Dosage & Administration
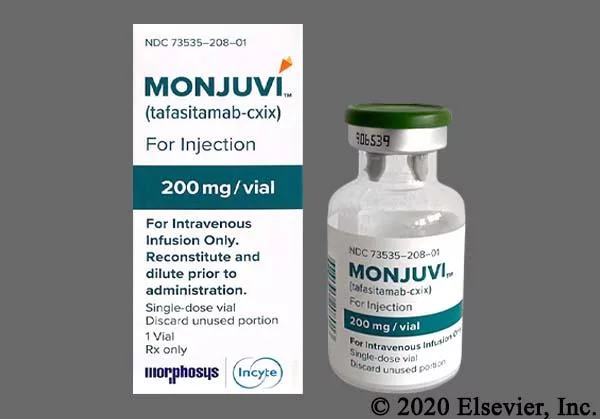
Monjuvi is supplied as a 200 mg lyophilized powder for reconstitution. The recommended dose is 12 mg/kg IV infusion, delivered on the following schedule:
- Cycle 1 – Days 1, 4, 8, 15, 22 (28‑day cycle).
- Cycles 2‑3 – Days 1, 8, 15, 22.
- Cycle 4 and beyond – Days 1 and 15 each 28‑day cycle.
Monjuvi is given in combination with lenalidomide (usually 25 mg orally on days 1‑21 of each cycle) for up to 12 cycles. After completing the combination phase, Monjuvi can continue as monotherapy until disease progression or unacceptable toxicity.
Premedication is essential: give an antihistamine, acetaminophen, and a corticosteroid (e.g., dexamethasone 20 mg) 30 minutes before the infusion. During the infusion, staff will monitor vitals every 15 minutes for the first hour, then every 30 minutes thereafter.
Monitoring & Follow‑up
Labs are drawn before each cycle to check CBC, liver enzymes, and renal function. If neutropenia drops below 500 µL, growth‑factor support (e.g., filgrastim) may be added, and the next dose might be delayed or reduced.
Imaging (PET/CT) is typically performed after cycle 3 and again at the end of cycle 12 to gauge response. Your oncologist will also assess any infusion reaction symptoms in real time and adjust the infusion rate if needed.
Support Resources
Feeling overwhelmed is normal. The manufacturer offers a patient‑discussion guide, financial assistance programs, and a 24‑hour nurse helpline. You can download the guide directly from the Monjuvi website or ask your clinic’s social worker for help.
Real World Stories
Data are powerful, but personal stories bring the numbers to life. Take Maggie, a 58‑year‑old mother of two from Ohio. After her DLBCL relapsed post‑R‑CHOP, her oncologist suggested Monjuvi based on the L‑MIND results. “I was nervous about another IV infusion,” Maggie told us, “but the staff walked me through each step, gave me the pre‑meds, and within a week I felt stronger.” Six months later, scans showed a complete response, and Maggie celebrated by taking a short road‑trip with her family—something she hadn’t been able to do in a year.
On the clinician side, Dr. Elena Rivera, a hematology‑oncology specialist in Boston, shared: “Monjuvi fills a critical gap for patients who can’t undergo stem‑cell transplant. The ability to combine it with rituximab and lenalidomide gives us a three‑pronged attack, and the safety profile, while requiring vigilance, is manageable in an outpatient setting.”
Stories like Maggie’s remind us that behind every trial statistic is a lived experience, full of hopes, fears, and triumphs. If you have a story to share, consider reaching out to a patient‑support group—your voice may help another person navigating the same journey.
Final Takeaway Summary
Monjuvi for lymphoma represents a meaningful advance for patients facing relapsed or refractory disease. The drug’s targeted mechanism, bolstered by lenalidomide and rituximab, can extend progression‑free survival dramatically while offering a tolerable safety profile when monitored carefully. However, it’s not a magic bullet; infusion reactions, myelosuppression, and infection risks demand close collaboration with your oncology team.
If you’re weighing Monjuvi as an option, ask your doctor about:
- Whether you meet the eligibility criteria (especially transplant ineligibility).
- The plan for pre‑medication and monitoring for infusion reactions.
- Supportive measures for neutropenia and infection prevention.
- Financial assistance programs that can offset out‑of‑pocket costs.
Remember, you’re not alone on this path—there are resources, experts, and fellow patients ready to help you make an informed decision. Stay curious, stay proactive, and keep the conversation open with your care team. Your journey may be challenging, but with the right information and support, you can navigate it with confidence.



















Leave a Reply
You must be logged in to post a comment.