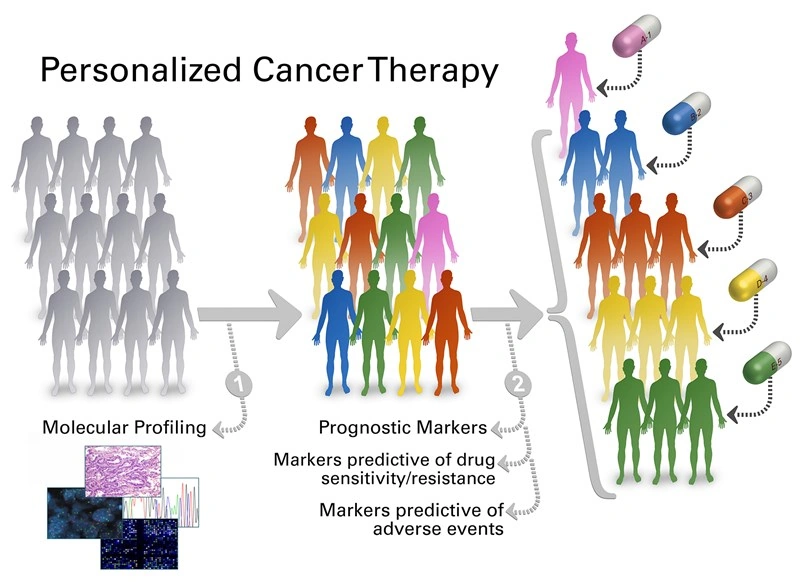
Most people think cancer treatment is a one‑size‑fits‑all prescription, but the reality is far more nuanced. Personalized cancer therapy tailors drugs to the unique genetic and molecular makeup of your tumor, aiming to hit the cancer where it’s vulnerable while sparing healthy cells.
In the next few minutes you’ll discover how this approach works, why it’s exciting (and sometimes tricky), and how cutting‑edge tools like urine tumor DNA testing are already reshaping everyday care—especially for bladder cancer. Ready? Let’s dive in.
How It Works
What’s the difference between “personalized” and “precision” medicine?
Both terms are tossed around in the media, yet they aren’t interchangeable. “Precision medicine” emphasizes the technical ability to pinpoint a molecular target—think a specific mutation in the EGFR gene. “Personalized cancer therapy” goes a step further: it blends that precision data with your overall health, lifestyle, and even personal preferences to craft a treatment plan that feels made just for you.
Why DNA, RNA, and the tumor micro‑environment matter
At its core, cancer is a genetic disease. Mutations in DNA drive uncontrolled growth; RNA tells the cell which proteins to build; and the surrounding micro‑environment (immune cells, blood vessels, signaling molecules) decides whether the tumor can thrive or be shut down. By sequencing the tumor’s DNA, measuring its RNA expression, and profiling the immune landscape, doctors can match you with drugs that exploit a specific weakness.
From biopsy to bedside: a simple flow
1️⃣ Your doctor orders a tissue or liquid biopsy.
2️⃣ A lab decodes the DNA and RNA, and flags actionable mutations.
3️⃣ A multidisciplinary team reviews the report, weighs your health history, and selects a therapy—often a targeted inhibitor, an immune checkpoint blocker, or a brand‑new mRNA vaccine.
4️⃣ You start treatment, and the team monitors response with repeat scans and, increasingly, non‑invasive tests like urine tumor DNA.
Real Benefits
Higher response rates and fewer side‑effects
When a drug hits a mutation that actually drives the cancer, the tumor shrinks faster and the treatment can be lower‑dose. A 2021 review in Cancers showed that patients on targeted therapies had a 30 % higher objective response rate compared with standard chemotherapy, while reporting significantly less nausea, hair loss, and fatigue.
Potential downsides you should know
Personalized therapy isn’t a magic bullet. Some hurdles still exist:
- Cost – Genetic sequencing and specialty drugs can be pricey, and insurance coverage varies.
- Access – Not every hospital offers a full panel of molecular tests.
- False positives – Occasionally a mutation looks “actionable” but the drug doesn’t work, leading to unnecessary treatment cycles.
Being aware of these trade‑offs lets you have an honest conversation with your oncologist about what’s realistic for you.
Chemo vs. Targeted/Immune Therapy: Quick Comparison
| Aspect | Standard Chemotherapy | Targeted / Immune Therapy |
|---|---|---|
| Mechanism | Poisons all rapidly dividing cells | Blocks a specific molecular driver or activates immune response |
| Response Rate | 20‑30 % | 40‑70 % (varies by tumor type) |
| Common Side‑effects | Nausea, hair loss, low blood counts | Skin rash, fatigue, endocrine changes (often milder) |
| Typical Duration | Cycles every 3‑4 weeks, often limited | Continuous until progression or intolerable toxicity |
A real‑life glimpse
Maria, a 58‑year‑old who survived a recurrence of bladder cancer, recounts how her oncologist suggested a urine‑based test instead of a painful cystoscopy. “When the result showed residual DNA, we switched my therapy before the cancer could grow again,” she says. Her story underscores how bladder cancer recurrence can be caught early, sparing patients from invasive procedures.
Cutting‑Edge Tools
mRNA vaccines – the newest weapon
Remember how mRNA technology powered COVID‑19 shots? Researchers have repurposed that platform to teach a patient’s immune system to recognize tumor‑specific antigens. Early trials show promising durability, especially when combined with checkpoint inhibitors. Think of it as a personalized “wanted poster” that trains your immune cells to hunt down the cancer.
Liquid biopsies: a non‑invasive window into your tumor
Instead of digging into tissue, doctors can now sniff cancer DNA floating in blood or urine. This is where urine tumor DNA shines—particularly for cancers that shed DNA into the urinary tract, like bladder cancer.
How utDNA testing catches bladder cancer recurrence early
Traditional surveillance relies on cystoscopy, a procedure many patients dread. A recent multicenter study found that utDNA testing identified recurrence a median of 8 weeks earlier than cystoscopy, with a specificity above 95 %.
Step‑by‑step guide to a utDNA test
1️⃣ Collect a fresh‑catch urine sample in a sterile container.
2️⃣ Ship the sample to a certified lab (most kits include prepaid mailing).
3️⃣ The lab extracts DNA, amplifies tumor‑specific markers, and runs a quantitative PCR.
4️⃣ You receive a concise report: “No detectable tumor DNA,” “Low‑level DNA detected – repeat in 4 weeks,” or “High‑level DNA – consider treatment adjustment.”
5️⃣ Your doctor integrates the result with imaging and symptom review to decide the next step.
Urine vs. Blood Liquid Biopsy: Quick Look
| Feature | Urine (utDNA) | Blood (ctDNA) |
|---|---|---|
| Best for | Bladder, kidney, prostate | Most solid tumors, hematologic cancers |
| Sensitivity | 70‑85 % (early stage) | 60‑80 % (depends on tumor burden) |
| Turnaround | 7‑10 days | 10‑14 days |
| Cost (US) | $150‑$250 | $300‑$500 |
| Invasiveness | Non‑invasive – just pee | Mini‑blood draw |
Bladder Cancer Case
Why bladder cancer is a perfect showcase
Bladder cancer has one of the highest recurrence rates of any solid tumor—up to 70 % within five years. Because tumor cells constantly shed DNA into urine, utDNA becomes a natural surveillance tool, aligning perfectly with the goals of personalized therapy.
Clinical evidence supporting utDNA
According to a 2023 trial published in Nature Medicine, patients monitored with utDNA experienced a 25 % reduction in time to recurrence detection compared with cystoscopy alone. The study’s authors—leaders in urologic oncology—concluded that “urine‑based liquid biopsies may become the new gold standard for post‑treatment surveillance.”
A patient’s journey, from surgery to tailored follow‑up
After a trans‑urethral resection, Alex (45) entered a monitoring program that combined quarterly cystoscopies with utDNA tests every two months. When a low‑level DNA signal appeared, his team adjusted his intravesical therapy before any tumor was visible on imaging. Six months later, Alex remains disease‑free and appreciates that he avoided an extra invasive procedure.
How doctors weave utDNA into bladder cancer treatment plans
Oncologists now use utDNA results to decide:
- Whether to intensify intravesical BCG therapy.
- If a checkpoint inhibitor is warranted for high‑risk molecular profiles.
- When to schedule the next cystoscopy, sparing patients unnecessary trips.
Future Outlook
Emerging research: next‑gen mRNA combos and AI‑driven matching
Scientists are engineering mRNA vaccines that encode multiple neo‑antigens tailored to an individual’s tumor—a “cocktail” that could boost immune recognition far beyond today’s single‑target shots. Meanwhile, artificial‑intelligence platforms are learning to predict which drug combinations will work best based on a patient’s full omics profile.
Regulatory shifts paving the way
The FDA’s recent guidance on “adaptive clinical trials” allows faster approval of therapies that demonstrate early signals of benefit in genetically defined sub‑populations. This regulatory flexibility means promising personalized drugs may reach patients sooner.
When will utDNA become standard of care?
Expert panels surveyed at the 2024 ESMO Congress forecast that by 2027, at least 80 % of U.S. oncology centers will use urine‑based liquid biopsies for bladder cancer surveillance. The timeline hinges on payer adoption and continued validation studies, but the momentum is undeniable.
What you can do today
Ask your oncologist:
- “Is my tumor being profiled for actionable mutations?”
- “Do we have access to urine tumor DNA testing for monitoring?”
- “What clinical trials might match my molecular profile?”
Taking an active role doesn’t just empower you; it nudges the whole system toward more precise, patient‑centered care.
Conclusion
Personalized cancer therapy isn’t a distant concept—it’s already changing how we treat, monitor, and even prevent disease. By matching your tumor’s unique genetic fingerprint to targeted drugs, mRNA vaccines, and ultra‑sensitive urine tumor DNA tests, doctors can strike harder and spare more of your healthy tissue. The benefits are clear: higher response rates, fewer side‑effects, and earlier detection of recurrence—especially in bladder cancer. Challenges remain, such as cost and equitable access, but the field is advancing rapidly, with AI‑driven drug matching and adaptive FDA pathways promising even faster progress.
If you’re navigating a cancer diagnosis, consider asking about molecular profiling and non‑invasive monitoring. The more you know, the more you can shape a treatment plan that truly feels personal. Together, we’re turning the tide—one tailored therapy at a time.

















Leave a Reply
You must be logged in to post a comment.