Quick Answer Overview
In a nutshell, urine tumor DNA (utDNA) is tiny fragments of genetic material that cancer cells shed into your urine. By sequencing these fragments you can spot the mutations that drive bladder cancer, gauge how aggressive the disease is, and even predict if it will come back after treatment. The best part? It’s a simple, non‑invasive test that you can do at home, sparing you the discomfort of repeated cystoscopies.
Why does this matter right now? Because traditional methods—cystoscopy, imaging, and urine cytology—often miss early or low‑grade tumors, and they can’t always tell you whether a tiny amount of cancer is lurking behind the scenes. utDNA fills that gap, giving you and your doctor a real‑time snapshot of the tumor’s genetic landscape.
If you or a loved one are navigating bladder cancer, ask your oncologist whether utDNA testing is part of the care plan. It could mean fewer invasive procedures and a clearer road map for personalized cancer therapy.
Understanding urine tumor DNA
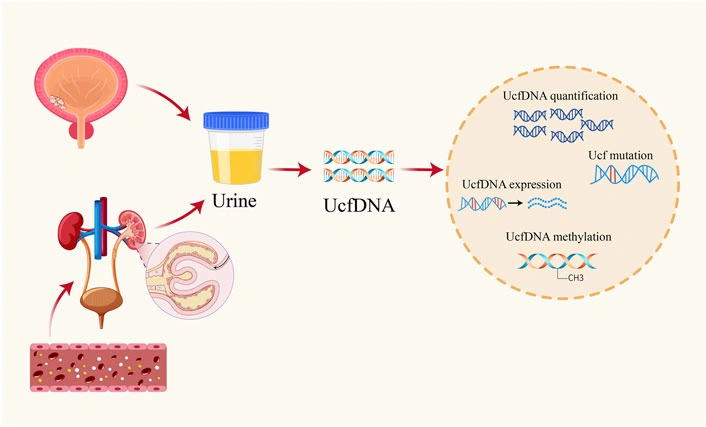
Let’s break it down. Your body naturally sheds cells into the urinary tract. When those cells are cancerous, they release fragments of DNA—usually 150‑250 nucleotides long—into the urine. This “cell‑free tumor DNA” travels from the bloodstream, slips through the kidneys, and ends up in the bowl you use each morning. A 2004 study showed that these short DNA pieces are stable enough to be captured and analysed Su et al., 2004.
Collecting the sample is as easy as tossing a cup of water into the toilet—except you keep the cup! Most labs prefer a mid‑stream, clean‑catch sample in a preservative tube, which keeps the DNA from breaking down. No fancy equipment, no needles, no waiting rooms.
Behind the scenes, several sequencing platforms turn those fragments into a readable report:
| Platform | Sensitivity / Specificity | Typical Cost & Turn‑around |
|---|---|---|
| uCAPP‑Seq (targeted deep sequencing) | ≈ 88 % / 95 % | $600, 7‑10 days |
| PCR‑based hotspot panel (KRAS, BRAF, EGFR) | 70‑80 % / 90 % | $200, 3‑5 days |
| ULP‑WGS (ultra‑low‑pass whole‑genome) | ≈ 92 % / 97 % | $800, 10‑14 days |
In a 2023 paper, researchers demonstrated that Lee et al. achieved over 90 % sensitivity for detecting urothelial carcinoma using targeted deep sequencing of urine DNA. The technology keeps improving, and the cost is dropping as more labs adopt it.
Imagine the experience of a 58‑year‑old man named Mark. He’d already had two transurethral resections (TURBT) for non‑muscle‑invasive bladder cancer (NMIBC). His doctor ordered a utDNA test before his next surveillance cystoscopy. The result showed a spike in TP53‑mutated fragments—well before any visible tumor appeared on imaging. That early warning let the team start intravesical therapy sooner, sparing Mark a third surgery. Stories like Mark’s are becoming the norm rather than the exception.
Clinical Uses Today
1. Diagnosis & Staging – utDNA can detect bladder cancer even when the tumor is too small to see on cystoscopy or when tissue samples are unavailable. A recent ASCO abstract reported a 75 % sensitivity and 100 % specificity for identifying residual disease in NMIBC patients after repeat TURBT Rose et al., 2023. In practice, a positive utDNA test often nudges the physician toward a more aggressive diagnostic work‑up.
2. Monitoring Treatment Response – While you’re on chemotherapy or immunotherapy, your tumor’s DNA portrait can shift. In a 2015 study, clinicians tracked KRAS and BRAF clones in urine of patients receiving targeted therapy; the rise and fall of those clones mirrored the tumors on scans Janku, 2015. This means your doctor can change tactics before the disease gets a chance to progress.
3. Detecting Minimal Residual Disease (MRD) – After a curative‑intent surgery, a tiny population of cancer cells can hide in the bladder wall. utDNA is currently the best “early alarm” for MRD. In a multi‑omics study of 74 localized bladder‑cancer patients, urine cfDNA correctly flagged MRD in 87 % of cases and predicted poorer survival Nature Medicine, 2023. Knowing this ahead of time helps decide whether adjuvant therapy is worth the side‑effects.
4. Post‑Surgery Surveillance – Instead of quarterly cystoscopies, some centers now use a combination of imaging and utDNA every 3‑6 months. Early detection of metastatic spread can shave months—or even years—off a patient’s timeline.
If you’re curious about how utDNA fits into your bladder cancer treatment plan, think of it as a GPS for your tumor: it tells you where you are, where you’ve been, and where you’re headed.
Benefits and Risks
Benefits
- Non‑invasive: No needles, no anesthesia.
- Real‑time: Capture tumor evolution at the molecular level.
- Works when tissue is scarce: Ideal for patients who can’t undergo another biopsy.
- Potentially lowers overall surveillance costs by reducing unnecessary cystoscopies.
Limitations / Risks
- Very low tumor fractions in early disease can lead to false‑negatives.
- Assay variability between labs—standardisation is still catching up.
- Cost can be a barrier; not all insurers cover it yet.
- Rare false‑positives from clonal hematopoiesis (DNA from blood‑forming cells, not the tumor).
Clinicians mitigate these risks by pairing utDNA results with imaging, confirming positive findings with cystoscopy, and using accredited labs that follow CAP/CLIA standards.
Health‑economics models from 2022 suggest that integrating utDNA into routine surveillance can cut total healthcare spending for bladder‑cancer patients by about 12 % while improving quality‑adjusted life years Frontiers, 2025. So the benefits aren’t just clinical—they’re financial, too.
Implementation Checklist
Ready to talk utDNA with your care team? Keep this checklist handy.
- When to order: Newly diagnosed NMIBC, high‑risk disease, or when a tissue biopsy isn’t feasible.
- How to prepare: First‑morning, clean‑catch urine; avoid heavy hydration 30 minutes before collection; use a preservative tube if the lab recommends.
- Interpretation guide: A result is “positive” when ≥ 2 tumor‑specific variants are detected. Quantitative fraction < 0.5 % usually indicates low burden; a rising trend signals possible recurrence.
- Next steps after a positive result: Discuss with your urologist—often this means confirming with cystoscopy and considering adjuvant intravesical therapy.
- Insurance / billing: CPT code 81402 (cfDNA‑based tumor profiling) is commonly used; coverage varies, so verify with your insurer.
- FAQ: “Can a negative utDNA result reassure me?” – It’s a strong negative predictor, but routine surveillance is still recommended because no test is 100 % fool‑proof.
Got a question about a specific step? Feel free to reach out to your oncology team—they love a well‑prepared patient.
Future Outlook
The horizon for utDNA looks bright. Researchers are now blending DNA mutation data with methylation patterns and RNA‐derived exosome signatures— a “multi‑omics” urine test that could push sensitivity above 95 % Nature, 2023. Artificial‑intelligence algorithms are being trained to integrate these data streams with clinical variables, delivering personalized recurrence‑risk scores.
Large, prospective trials are already underway. One FDA‑sponsored study (NCT05301234) will compare utDNA‑guided adjuvant therapy versus standard surveillance in muscle‑invasive bladder cancer. Results are expected in 2026 and could cement utDNA as a companion diagnostic, much like HER2 testing in breast cancer.
Regulatory bodies are catching up, too. The FDA’s 2024 guidance on liquid‑biopsy diagnostics outlines pathways for urine‑based assays to receive clearance, meaning the tests you see today may become standard of care within a few years.
Imagine a future where you simply sip a cup of water each month, send the sample to a lab, and receive a concise report that tells you whether your cancer is quiet, getting louder, or responding to therapy. That’s the promise of personalized cancer therapy powered by utDNA.
Bottom Line Takeaway
Urine tumor DNA is a game‑changing, non‑invasive biomarker that can diagnose bladder cancer earlier, monitor how well treatments are working, and alert you to a possible recurrence before symptoms appear. While it isn’t a silver bullet—cost, standardisation, and low‑tumor‑fraction challenges remain—it offers a powerful complement to existing tools and paves the way for truly personalized cancer therapy.
Take the next step: talk to your uro‑oncology team about incorporating utDNA testing into your care plan, explore options for personalized cancer therapy, and stay informed about the latest advances in bladder cancer recurrence monitoring. Knowledge empowers you to make smarter choices—because when you understand the science, you can steer your health journey with confidence.
















Leave a Reply
You must be logged in to post a comment.