Gene therapy is looking like a game-changer for sickle cell. Imagine it going straight to the source, fixing the DNA-level mess causing those painful, sticky red blood cells. Yes, it could reduce stroke risk by restoring normal brain blood flow. This isn’t just theory. Early clinical trial results (more about those later) show staying power for at least 1–2 years. Big teams at St. Jude have given it the green light.
But hold up—when the brain’s involved, the stakes are high. Sickle cell patients often face brain ischemia, where cells clog up the system. Until recently, treatments like hydroxyurea barely dented this risk. Gene therapy’s offer isn’t a patch. It’s reprogramming your blood from the inside. Let’s walk through what real patients are already experiencing.
Real or hype? Let’s break it down
Okay, let’s act like this isn’t some 2050 tech. The FDA gave the thumbs-up to Casgevy and Lyfgenia in December 2023. Yep, each costs over $2.95 million. But here’s the deal: these treatments aren’t patches. They’re rewriting the body’s blueprint, offering something that even hydroxyurea can’t—a shot at a durable fresh start.
| Therapy | Mechanism | Key Benefit |
| Casgevy | CRISPR DNA “scissors” reactivate fetal hemoglobin | Cutting off sickling like a bouncer at the DNA door |
| Lyfgenia | Lentivirus truck delivers working hemoglobin gene | Integrated software update for red blood cells |
How’s blood flow tied to strokes?
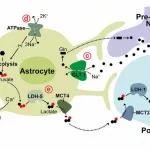
In your average person, brain blood flow’s smooth sailing. For sickle cell patients, those cells gather like traffic at a concert. These rigid little cells ram into vessel walls, speeding their way into pressure overload. Doctors nickname this turbulent jets—fast flickering that poses as normal. Keep it up, and brain ischemia treatment becomes your top priority.
Gene therapy’s impact on this mess
St. Jude’s 2025 paper shows first-time blood flow normalization. Normal—get that word? These patients, after gene therapy, didn’t end up with stroke-risk zones. Casgevy and Lyfgenia tackle the hemoglobin problem differently, but both are kind of DNA Wonder Women here. Casgevy knocks out the stop signal for fetal production. Lyfgenia shoehorns the correct gene into your cells. Either way, you’re looking at less “sticking,” more gliding.
Wait, but just three patients? Reliable?
Suspicious eyebrow raise, 100%. Early signals in medical science never sleep well-known. But check this out: these weren’t random. They’d been dodging transfusions and staring down repeat strokes for years. Gene therapy elevated their red blood cell performance, greenlit smoother flow and—huge bonus—they’ve shown stable results for over a year. Bottom line: small sample, but fireworks-size impact.
Gene editing vs gene addition
You’re probably wondering: “But which ology is more my jam?” Both aim for less OC rush and fewer bedsores. But they work under different legal tracks. Approved by the FDA for patients 12+ with a history of the toughest sickle events, these aren’t quick fixes. They’re _YOUR SHOULDERS REMEMBER THE PRICKING_ energy.
Lyfgenia: The mRNA delivery mule
RNA biologists came up with a lentiviral delivery vehicle (think of it like a genetically-engineered Trojan horse). This “truck” carries a functional hemoglobin gene and parks it in your stem cells’ garage. Challenge: Your body might not roll out the red carpet. Chemotherapy is used to clear receptors—a tough price.
Casgevy—DNA’s favorite editor
You’ve heard of flossing your teeth, right? Casgevy’s flossing your DNA. It cuts a gene (_BCL11A*) in your stem cells—think of it as hitting CMD+Z on sickle cell production. Your red blood cells now rewind 12% fetal hemoglobin, which is like throwing a water balloon at flames.
Sprint or marathon? Let’s put it on a clock
Traditional approaches are like a single-speed fixie you ride around the block: hydroxyurea >30% effectiveness in maintaining baseline flow. Blood transfusions? Run, fade, repeat.
Gene therapies, meanwhile, are like F-16s. Even ~100 programmed red cells flood your bloodstream with “F” values, turning emergency rooms into last year’s story. Patient001 (real human, heard of) from pharmacytimes tells it like it happened: “NP-checkups. No pain meds. It was night and day.”
So, what about me? Cost, access reality bites
You’re not crazy—as much as we’d love to all get a Rip Van Winkle edit under the Christmas tree. Casgevy and Lyfgenia’s price tag: $2.95 million a pop. Studies report insurers are playing hardball—everywhere buffer. But here’s a truth bomb: money’s not the only wall here. Many states still haven’t licensed facilities for infusing these treatments.
Patients’ emotional whiplash
Take Example Patient 001. Before treatment, they’d mapped their pharmacy windows like a bad dream. Troughs of pain, talks with specialists, and ICU stays made their life a no man’s land. Gene therapy didn’t solve existential dread, but bones went still, nights were less relentless, and sun block came off their calendar (metaphor if you’re not a prior).
“The first time I realized I didn’t reach for my rescue meds after 3am?” Wild. Imagine talking to your GP: “Normal blood flow + fewer crises? Sign me up—and draft a rocket.” It’s real madness-fighting madness. But the numbers… they tilt toward hope.
The un-pretty side
We’re not spinning fairy tales. No deus ex machina without some gear-shift chaos. Bone marrow prep? Classic fever dreams. Steroids, chemo highs—it’s a tough ride the first month. And we still don’t know what this will do in 5 or 10 years. Even the coolest data admits that: Blood Advances quietly coughs up, “More runway needed.”
So, what’s stopping you from trying this?
Here’s what folks are asking before shedding the sickle curse:
Am I eligible? The swipe left/right
First rule: ≥12 years old. Second: you’ve got recurrent crises, or allied symptoms that resemble rollercoasters. St. Jude, Children’s National, and Mount Sinai are offering this treatment—slowly. Best tip? Make friends with _your clinic sickle cell squad_ and ask point-blank: “When can I get optimized with this, Doc?”
The 2023 timeline and what it really means
December 8, 2023—after a decade’s worth of work, the FDA made their decision. Lyfgenia and Casgevy entered the “How We Got Here” section. But while this was a milestone, it’s not Everest summit vibes yet. Curing SCD? Still 3D chess.
What you won’t hear in hype reels
Even gold-standard care (transplants through matched siblings!) works faster than gene edits. Casgevy and Lyfgenia require a serious bone marrow rehab process. Blood transfusions and stem cell harvests? Not quite movie nights. But for people terrified of repeat strokes—or terrified of seeing their kids suffer—it’s a gamble with better odds than before.
All the good, minus the red flags
Gene therapy promises blood flow resets, smoother lives, and a better future. But it’s not a perfect solution. From cost to accessibility, there’s a lot to navigate. Still, the breakthrough is undeniable. The future of sickle cell treatment is unfolding, and if you’re in the game, there’s hope that one day, this could be your reality too.
What comes after this wild dash?
We’re starting to discuss extending a lifeline to underserved populations. Implementing similar edits with gene writing (NOT cutting)? It’ll be here. If you’re watching from the SCD sidelines right now—stick your head out the window. We’ve got two FDA-approved therapies, more pipelines queued, and an entire generation of patients raising their hands saying “I’ll try that.”
Wrap-up: SCD, Stroke Risk, and gene therapy’s underdog story
The truth is sickle cell ran circles around even the savviest treatments, sucking brain oxygen like it was punchline. Gene therapy isn’t the Avengers. It’s more like that friend with a wrench you didn’t think could fix your Saturn’s engine—but YOU GET TO DRIVE.
Here’s what I want you chewing on: brain ischemia, noisy red cell highways, and all the stale info doctors pushed last decade is getting nutritional refresh courtesy Casgevy and Lyfgenia. We’ve got strokes getting sidetracked by punchy RBCs, long-term stabilization hints, and particles dancing to gene-level rhythms you didn’t dare hope for last year. Reach out to your marrow bank. Keep mounting the fight. Could you be next? That’s what we’re writing those chapters for.
Last tidbit? The field’s still up in the air after 30 years. The American Journal of Hematology finally lets the air out: patients are seeing good brain flow after these edits. Not _cure_ by any means. But _cured enough_ to start loading the car for road trips again? One hiccup into this treatment, and maybe dealing with magnetic resonance imaging less like ankle weights, and more like flu shots.
Do you vibe with that? Or would you rather ride waves of pain meds and slippery clinics? Keep a bad flavor note—ask the hard questions. But better—scan those eligibility docs alongside your SCD ecosystem now. The future’s in bold, and your time’s vast.















Leave a Reply
You must be logged in to post a comment.