When you’re staring at a prescription bottle labeled Mavyret, the excitement of finally having a cure for hepatitis C can be mixed with a little anxiety. “Will something show up weeks or months later?” is a question many of us have asked. The good news is that Mavyret’s safety profile is excellent, but a handful of serious, sometimes delayed, reactions can happen—especially hepatitis B re‑activation and liver decompensation. Below is a friendly, no‑jargon walk‑through of what you need to watch, why it matters, and how to stay safe while you enjoy a virus‑free life.
Quick Summary Overview
- HBV re‑activation – Can surface months after finishing treatment and may lead to severe hepatitis, liver failure, or even death if not caught early.
- Hepatic decompensation – Rare but possible, especially in people with compensated cirrhosis or portal hypertension; symptoms may appear during therapy or up to three months after stopping.
- Persistent fatigue, itching, or jaundice – Signs that warrant lab checks even if they seem mild.
- Very rare renal or skin issues – Isolated case reports, usually reversible when identified.
Knowing these risks, getting the right labs, and listening to your body can keep the cure safe and give you peace of mind.
How Mavyret Works
Mechanism of Action
Mavyret combines two powerful antivirals:
- Glecaprevir – an NS3/4A protease inhibitor that blocks a key viral enzyme.
- Pibrentasvir – an NS5A inhibitor that stops viral RNA replication.
This two‑pronged attack shuts down hepatitis C in virtually every genotype (1‑6) with an eight‑week course for most patients.
Typical Treatment Course
Most adults and children ≥ 3 years take one tablet daily for eight weeks. Some genotypes or previous treatment failures need a twelve‑week regimen. For exact dosing schedules, see our Mavyret dosing guide.
Short‑Term Side Effects (Why They’re Not the Focus)
Common Reactions (≥ 10 %)
During the eight‑week window, about one in ten people notice headache or tiredness. Diarrhea, nausea, and mild itching can pop up, too, but they usually fade as your body adjusts.
How Short‑Term Data Informs Long‑Term Safety
Across 13 clinical trials involving roughly 2,300 adults, the overall discontinuation rate because of adverse reactions was only 0.1 %. Most reactions were mild, and just one serious adverse reaction was reported. This sturdy safety foundation helps doctors feel confident monitoring for the rarer, long‑term issues we’ll discuss next.
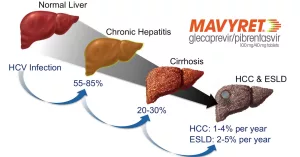
Long‑Term Side Effects – In‑Depth
Hepatitis B Reactivation
Even if you never had active hepatitis B, a past infection can lurk silently. When Mavyret clears hepatitis C, the immune balance shifts, and HBV can awaken.
- Incidence & severity: Post‑marketing surveillance has identified cases that progressed to fulminant hepatitis, liver failure, and, in extreme situations, death.
- Risk factors: Prior HBV exposure, immunosuppression, or concurrent antiviral therapy can tip the scales.
- Monitoring: Test for HBV DNA before starting Mavyret, then repeat every four weeks for the first three months after therapy ends.
Real‑World Snapshot
Mark, a 52‑year‑old from Ohio, finished his eight‑week Mavyret course without a hitch. Two months later, he felt unusually jaundiced and fatigued. A simple blood test revealed rising HBV DNA, and his doctor started tenofovir immediately. Mark’s liver function bounced back, and he’s now hepatitis B‑controlled. Stories like Mark’s illustrate why a quick HBV screen is non‑negotiable.
Management Tips
If re‑activation is caught early, clinicians usually add a nucleos(t)ide analogue such as tenofovir or entecavir. The key is vigilance—don’t dismiss a new rash or a splash of yellow in the eyes.
Hepatic Decompensation / Liver Failure
While Mavyret is safe for most with compensated cirrhosis, rare post‑marketing cases have shown decompensation, especially in patients with portal hypertension or prior decompensation events.
- Onset: Median time to first sign was 27 days, but cases have appeared up to three months after stopping.
- Red‑flag symptoms: New jaundice, swelling in the abdomen (ascites), confusion (hepatic encephalopathy), or sudden bleeding from varices.
- Action: Immediate liver panel (ALT, AST, bilirubin, INR). If decompensation is confirmed, discontinue Mavyret and refer to a hepatologist.
Lab‑Monitoring Schedule
| Time Point | Tests |
|---|---|
| Baseline | ALT, AST, Bilirubin, INR, CBC, HBV DNA |
| Week 2 | ALT, AST, Bilirubin |
| Week 4 | ALT, AST, Bilirubin, INR |
| End‑of‑Treatment | Full liver panel + HBV DNA |
| 4 Weeks Post‑Tx | Full liver panel + HBV DNA |
| 12 Weeks Post‑Tx | Full liver panel (optional if stable) |
Renal Impairment (Rare)
A handful of case reports describe a temporary rise in creatinine during or after therapy. Baseline kidney function is advisable, especially for patients on nephrotoxic meds.
Dermatologic / Immunologic Reactions (Late‑Onset)
Occasionally, hives, angio‑edema, or a persistent rash can emerge weeks after the last dose. These are immune‑mediated and usually respond to antihistamines or a short steroid burst.
Who Is Most at Risk?
- Anyone with a documented past HBV infection.
- Patients with compensated cirrhosis, especially those with portal hypertension.
- People on medication‑assisted treatment (MAT) or who inject drugs—clinical trials noted higher adverse‑reaction rates in these groups.
Pre‑Treatment Checklist
- HBV surface antigen and core antibody testing.
- Baseline liver function tests and ultrasound if cirrhosis is suspected.
- Review current meds – avoid atazanavir, rifampin, or other known interactions.
- Discuss alcohol use (keep it low) and any herbal supplements.
Managing & Monitoring Long‑Term Risks
Laboratory Follow‑Up Plan
Stick to the schedule in the table above. Most labs can be drawn at your primary‑care office or a local lab. Bring the results to your hepatitis C specialist so they can spot trends early.
Patient‑Education Checklist
Print or save a simple one‑page cheat sheet:
- Watch for yellow eyes, dark urine, or severe fatigue.
- Note any new rash, itching, or swelling of the face/lips.
- Call your doctor right away if any red‑flag symptom appears.
- Keep a medication diary—write down the date, dose, and any side effect.
- Schedule a follow‑up appointment 4 weeks after treatment ends.
When to Involve a Specialist
If labs show a sudden rise in bilirubin > 2 mg/dL, INR > 1.5, or if HBV DNA spikes, a hepatologist or infectious‑disease doctor should take the lead.
Mavyret vs. Other Pan‑Genotypic Regimens
| Regimen | Treatment Length | SVR Rate | Major Long‑Term Risks | Typical Cost (US) |
|---|---|---|---|---|
| Mavyret | 8–12 weeks | 95‑99 % | HBV re‑activation, rare hepatic decompensation | See Mavyret cost |
| Epclusa | 12 weeks | 94‑98 % | Elevated bilirubin, drug‑drug interactions | Comparable, varies by insurance |
| Harvoni | 8–12 weeks | 94‑97 % | Low long‑term risk, but higher pill burden | Generally higher than Mavyret |
If you’re curious how Mavyret stacks up against Epclusa, check out our side‑by‑side comparison here.
Bottom Line & Call‑to‑Action
Mavyret delivers one of the highest cure rates in hepatitis C therapy, and for the overwhelming majority the journey is smooth. The long‑term side effects you need to keep on your radar are hepatitis B re‑activation and, in a tiny subset, hepatic decompensation. By getting a baseline HBV screen, following a simple lab schedule, and staying alert to new symptoms, you can protect yourself while enjoying the freedom of a virus‑free life.
If you’re about to start Mavyret or have just finished, ask your provider for a complete HBV check‑up, keep a symptom diary, and set up the follow‑up labs we outlined. For more details on what to expect after you stop the medication, read about Mavyret side effects after stopping. Want a quick refresher on the usual side effects? Our Mavyret side effects page has a concise list. And if you’re still wondering what is Mavyret used for, you’ll find the answer in plain language there.
Take charge of your health—knowledge is the best companion on the road to cure.




















Leave a Reply
You must be logged in to post a comment.