Is there a mavyret generic? Short answer: No – the brand‑name drug Mavyret (glecaprevir/pibrentasvir) has no FDA‑approved generic equivalent in the United States as of 2025. The longer answer is that the lack of a generic version means patients and providers must navigate the price of the brand name, insurance formularies, and patient‑assistance programs to get the treatment they need.
Quick Answer
If you’re wondering whether you can pick up a cheap generic version at your local pharmacy, the answer is still “not yet.” The drug’s patents are still in force, and the only legal way to obtain it in the U.S. is the branded product from AbbVie. That said, the conversation doesn’t end there – we can still talk about cost‑saving options, the science behind the drug, and how it stacks up against alternatives like Epclusa.
Patent Landscape
Patents are the invisible walls that keep a drug exclusive. For Mavyret, the key U.S. patent (Patent 10,028,937) protecting the antiviral compounds expires on June 10 2030. Until then, no manufacturer can submit a generic application that the FDA will approve as therapeutically equivalent.
According to Drugs.com, “No therapeutically equivalent version of Mavyret is available in the United States.” Internationally, some regions have “generic‑like” versions under different branding, but they are not approved for U.S. patients, and importing them can be risky.
Mavyret Cost
The price tag on Mavyret is a big part of why many people hope for a generic. A typical 8‑week course (84 tablets) can range from $28,000 to $35,000 in wholesale pricing, translating to an out‑of‑pocket cost that varies wildly depending on insurance coverage.
AbbVie offers a Mavyret Savings Card and many Medicare Part D plans list the drug on their formulary with varying copays. If you’re uninsured, speak with your provider about enrollment in patient‑assistance programs – they can sometimes slash the price by more than 50 %.
For a deeper dive on price‑saving strategies, see our Mavyret cost guide.
Dosing Guide
Understanding the dosing schedule helps you stay on track and avoid missed doses, which could jeopardize a cure.
- Adults (including teens ≥ 3 years): three tablets taken once daily with food. Each tablet contains glecaprevir 100 mg + pibrentasvir 40 mg, for a total daily dose of 300 mg/120 mg.
- Pediatrics (3 years – 12 years or weight ≥ 45 kg): the same three‑tablet regimen is recommended, but some clinicians adjust based on weight.
- Special populations: patients with moderate renal impairment (eGFR 30‑59 ml/min) can still use the standard dose, but those on dialysis should discuss dosage with a specialist.
For the full dosing chart, check out our Mavyret dosing article.
What Is Mavyret Used For
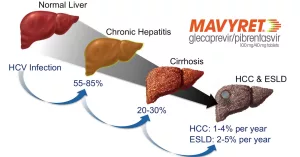
Mavyret is a powerhouse against chronic hepatitis C (HCV). It works on all six major genotypes (1‑6), whether you have no cirrhosis or compensated cirrhosis. The drug is also approved for patients who have previously been treated with an NS5A inhibitor or an NS3/4A protease inhibitor, but not both.
Typical treatment durations:
- 8 weeks – most treatment‑naïve patients without cirrhosis.
- 12 weeks – patients with compensated cirrhosis or certain prior‑treatment histories.
- 16 weeks – those with more complex prior therapy or certain genotype‑3 cases.
Side Effects Overview
Every medication has a trade‑off, and Mavyret is no exception. The most common side effects (affecting ≥ 10 % of users) are:
- Headache
- Fatigue (tiredness)
- Diarrhea
Serious but rare events include elevations in liver enzymes, reactivation of hepatitis B in co‑infected patients, and drug‑drug interactions with strong CYP3A inducers.
Long‑term safety data are reassuring; clinical trials report cure rates of 95‑99 % with minimal chronic toxicity. For detailed adverse‑reaction information, visit our Mavyret side effects page.
When you finish treatment, most people feel fine, but a few notice lingering fatigue or mild abdominal discomfort. To learn more about what can happen after stopping, see mavyret side effects after stopping.
According to a study published on NCBI’s LiverTox, the drug’s safety profile remains favorable even in patients with compensated cirrhosis, provided they are monitored regularly.
What To Avoid
Because Mavyret is metabolized by the liver’s CYP3A pathway, certain substances can either blunt its effectiveness or boost its toxicity.
- Avoid strong CYP3A inducers such as rifampin, St. John’s wort, and certain anticonvulsants.
- Limit grapefruit and grapefruit juice – they can raise drug levels and cause unexpected side effects.
- Inform your doctor about any over‑the‑counter supplements; vitamins that contain high doses of St. John’s wort are especially problematic.
For a comprehensive list, read our what to avoid when taking mavyret guide.
Mavyret vs Epclusa
| Feature | Mavyret (glecaprevir/pibrentasvir) | Epclusa (sofosbuvir/velpatasvir) |
|---|---|---|
| Generic availability (U.S.) | No generic version | Generic 400 mg/100 mg tablets exist |
| Typical treatment length | 8 – 16 weeks (genotype‑specific) | 12 weeks for all genotypes |
| Pill burden | 3 tablets daily | 1 tablet daily |
| 2025 cost (brand) | ≈ $30,000 per course | ≈ $25,000 (generic) per course |
| Key contraindications | Strong CYP3A inducers | Severe renal impairment (eGFR < 30) |
If you’re trying to decide which regimen fits your lifestyle, check out our detailed comparison at Mavyret vs Epclusa. The table above is a quick snapshot, but your doctor will tailor the choice to your genotype, liver status, and any co‑medications.
Real‑World Experience
Let me share a quick story from a friend of mine, “Laura.” She’s a 58‑year‑old accountant diagnosed with genotype 1 a HCV a few months ago. Her doctor prescribed an 8‑week Mavyret course. Laura was nervous about the price, but after enrolling in AbbVie’s savings program, her out‑of‑pocket cost dropped to about $2,000.
She took the three tablets each morning with breakfast, set a phone alarm, and never missed a dose. By week 8, she felt the usual fatigue was gone, and a post‑treatment blood test confirmed “SVR12” – meaning the virus was undetectable 12 weeks after finishing therapy. She told me, “It felt like finally getting my life back.” Her experience underscores two things: adherence matters, and the right financial support can make a life‑saving treatment affordable.
From a clinical perspective, hepatologists echo Laura’s story. A recent AASLD 2024 guideline (AASLD 2024 Guidelines) emphasizes that the cure rates for Mavyret remain >95 % when patients complete the prescribed regimen, regardless of genotype.
Final Thoughts
So, where does that leave us? While a true mavyret generic isn’t on the shelves yet, you don’t have to feel powerless. Knowing the patent timeline, exploring cost‑saving programs, understanding the dosing schedule, and being aware of side effects and drug interactions can all empower you to make the best decision for your health.
Remember, the journey to a hepatitis C cure is a partnership between you, your doctor, and the support resources around you. If you have questions about pricing, dosing, side effects, or how Mavyret stacks up against other treatments, don’t hesitate to bring them up at your next appointment. And if you’ve already walked this path, sharing your experience can help someone else feel less alone.
We’ve covered a lot, but the most important takeaway is this: cure is possible, and with the right information you can navigate the system confidently. Stay curious, stay proactive, and know that you’re not alone in this.


















Leave a Reply
You must be logged in to post a comment.