Imagine being able to see not just the shape of a brain, but how it’s actually working –‑ like watching traffic flow on a city map instead of just looking at the streets. That’s what modern brain scan diagnosis does for dementia, and it’s changing the conversation from “when will it happen?” to “how can we catch it early and act now.”
In the next few minutes I’ll walk you through why this matters, the different scanners you might hear about, the AI tools that are turning raw images into actionable insights, and how a small trial in Kent is already showing results. Think of it as a friendly coffee‑chat where we demystify a high‑tech topic that could one day help a loved one you care about.
Why It Matters
What does “brain scan diagnosis” actually mean?
Traditional imaging – CT or MRI – shows the brain’s structure, kind of like a photograph of a house. A brain scan diagnosis goes a step further and reveals function: blood flow, metabolism, and neural activity. In other words, we can see which rooms in that house have lights on and which are dark, giving clinicians clues long before holes appear in the walls.
How is it different from a regular MRI/CT?
Standard scans tell us whether something is broken; functional scans tell us whether it’s working properly. For dementia, the early changes are often metabolic or vascular, invisible on a structural scan.
According to a 2021 overview of SPECT imaging, “SPECT makes brain activity visible, allowing clinicians to pinpoint which part of the brain is under‑performing”.
Why is it a game‑changer for dementia and Alzheimer’s?
Early physiological markers—like reduced blood flow in the posterior cingulate—can appear years before memory loss becomes obvious. A recent study in Nature Medicine used AI on functional MRI to split depression and anxiety into six sub‑types, a method now being adapted for Alzheimer’s to identify distinct disease pathways.. The takeaway? We can target treatment to the right “brain‑type” instead of a one‑size‑fits‑all approach.
Scan Types Explained
SPECT (Single‑Photon Emission Computed Tomography)
SPECT shows how blood carries a tiny radioactive tracer through the brain. The resulting images highlight active versus sluggish regions, making it perfect for spotting early dementia patterns.
In a real‑world example, a patient named “Nancy” (anonymized for privacy) underwent a SPECT scan that revealed a subtle perfusion drop in the temporal lobes. This early flag prompted lifestyle changes and a medication adjustment that slowed her cognitive decline.
PET (Positron Emission Tomography)
PET uses radiolabeled sugars or amyloid‑binding compounds to map metabolism or protein build‑up. Amyloid PET is currently the gold standard for confirming Alzheimer’s pathology, while FDG‑PET assesses overall brain glucose use.
qEEG / Brain‑Mapping
Quantitative EEG records electrical activity with a headset and turns the data into a “brain map.” An AI engine then compares the pattern to thousands of known biomarkers. The Nickerson Institute reports that a six‑minute qEEG scan can detect nine common mental‑health conditions, including early signs of dementia, with results delivered in about 15 minutes..
Emerging AI‑enhanced MRI
Researchers are feeding functional MRI data into deep‑learning models that automatically highlight regions of reduced connectivity. The same AI that clustered depression sub‑types is now being trained on Alzheimer’s datasets, promising faster, more precise diagnostics.
| Modality | What It Shows | Typical Cost (UK) | Result Time |
|---|---|---|---|
| SPECT | Blood flow & functional perfusion | £250‑£500 | 15‑30 min (pre‑read) |
| PET (Amyloid) | Amyloid/tau plaques | £1,200‑£1,800 | 1‑2 days |
| qEEG | Electrical activity patterns | £150‑£300 | 15 min |
| AI‑MRI | Connectivity & metabolism | £800‑£1,200 | Same‑day |
AI Tools Emerging
What is “AI dementia detection”?
In practice, a scan is uploaded to a secure cloud where a trained neural network evaluates thousands of voxels (3‑D pixels). The algorithm spits out a risk score, a probability map, and sometimes a suggested dementia subtype.
AI dementia detection tools are already being piloted in several NHS trusts, offering clinicians a second opinion that’s based on data rather than gut feeling.
The “Alzheimer’s AI tool” that’s making headlines
The Kent portable scanner trial used a proprietary AI platform that combines SPECT data with a deep‑learning classifier. Early results showed a sensitivity of 84 % for moderate‑to‑severe Alzheimer’s and a specificity of 78 % for healthy controls.
Read more about the technology in our Alzheimer’s AI tool page.
How AI helps identify dementia types
Traditional diagnosis groups dementia into a handful of categories: Alzheimer’s, vascular, Lewy‑body, frontotemporal, etc. AI can tease out subtle patterns—a slight perfusion dip in the occipital lobe might point to Lewy‑body, while diffuse frontal hypoperfusion leans toward frontotemporal.
Our dementia types identification guide breaks down these signals in plain language.
Ethical and trust considerations
Transparency matters. Good AI tools show the raw heat‑maps that clinicians can review, and they store data under strict GDPR controls. The final diagnosis always rests with a qualified neurologist—AI is a powerful assistant, not a replacement.
Benefits vs Risks
Benefits of brain‑scan diagnosis
- Fast, objective insight – reduces months of uncertainty.
- Personalized treatment plans – match medication to the brain’s actual deficits.
- Potential to delay progression – early lifestyle and pharmacologic interventions work best when started early.
Potential risks & limitations
- False positives can cause unnecessary anxiety.
- Not every clinic has the technology or expertise to interpret functional scans.
- Cost and insurance coverage can be variable.
When a scan isn’t needed
Guidelines from the American Academy of Neurology advise against routine imaging for uncomplicated dementia when the clinical picture is clear. A scan is most valuable when the diagnosis is uncertain or when atypical features appear.
For example, a 2019 article on bipolar disorder stresses that “no scan can diagnose bipolar directly,” underscoring the importance of clinical assessment first..
Kent Trial Insights
Trial design and who was scanned
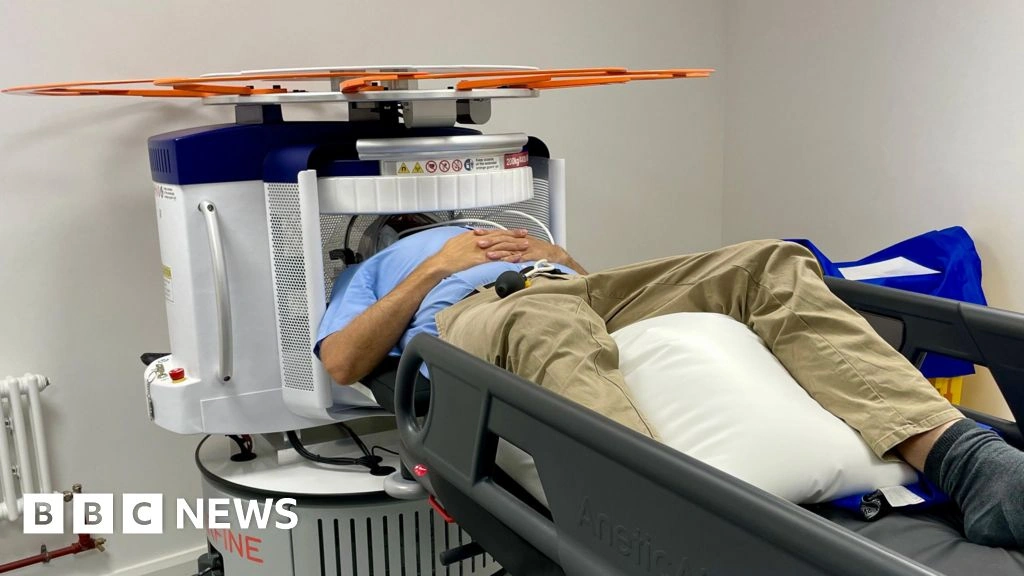
The Kent study recruited 300 participants from primary‑care practices, ages 55‑80, with mild memory complaints but no formal dementia diagnosis. A portable SPECT scanner was set up in community health centres, allowing a “walk‑in” model.
Early results: speed and satisfaction
Patients received a preliminary functional report in under 30 minutes. Over 90 % said the quick feedback reduced their stress, and clinicians reported a 30 % reduction in diagnostic “guesswork.”
What the radiologist said
“Standard scans show brain structure, but these newer tests reveal how the brain is actually functioning,” said SMH radiologist Richard Hickson during a press briefing. This sentiment captures why functional imaging feels like turning the lights on in a dark room.
Implications for nationwide rollout
If the portable model proves cost‑effective, it could be deployed in rural GP practices, reducing travel barriers and catching dementia earlier across the UK.
Getting a Scan
How to talk to your doctor
Bring a simple list:
- Your main concerns (e.g., “I’m forgetting recent conversations.”)
- Family history of dementia or Alzheimer’s.
- Any recent head injuries or strokes.
Ask directly: “Is a functional brain scan appropriate for my situation?” If you’re interested in the newest AI‑driven options, you can mention the Kent trial or ask about a SPECT scan.
What to expect on the day
- Preparation: No metal, light clothing, and a brief health questionnaire.
- Duration: Most functional scans take 10‑20 minutes; the AI analysis may add another 10‑15 minutes.
- Safety: Radiation dose from a SPECT scan is comparable to a few months of natural background exposure.
Reading the report
Key terms you’ll see:
- Perfusion deficit – area with reduced blood flow.
- Standardized Uptake Value (SUV) – how much tracer a region absorbed.
- AI risk score – a numeric probability of dementia, usually expressed as a percentage.
Next steps after results
If the scan shows early changes, your clinician may suggest:
- Targeted cognitive training or lifestyle programs.
- Medication that boosts cholinergic function or improves blood flow.
- Enrollment in a clinical trial – many are now seeking participants with early SPECT findings.
For more on how AI tools can guide treatment, explore our early dementia detection article.
Practical Next Steps
Take action today
Even if you’re not ready for a scan, you can start with simple brain‑healthy habits: regular aerobic exercise, a Mediterranean‑style diet, and mentally stimulating activities like learning a new language.
Stay informed
Technology moves fast. Sign up for newsletters from reputable neurology societies, and keep an eye on local health‑board announcements for portable‑scanner sessions.
Share your story
If you or someone you love has undergone a brain scan, consider discussing the experience with your doctor and, if comfortable, with support groups. Real‑world anecdotes help clinicians refine guidelines and improve future care.
In a world where dementia often feels like a looming, invisible threat, brain scan diagnosis offers a tangible way to see what’s happening inside the skull. It’s not a crystal ball, but it’s a powerful lens that can bring clarity, hope, and earlier intervention. If you’re curious, ask your GP about functional imaging options today – you might just catch the first flicker of change before it becomes a flame.
















Leave a Reply
You must be logged in to post a comment.