Here’s the thing—the NHS wants every baby born in the UK to have their entire genome sequenced by 2030. It sounds like sci-fi made real, right? But here’s the kicker: genetic testing in newborns isn’t just about catching conditions early. It’s a tangled maze of hope, hype, and heart-wrenching dilemmas.
Picture this: you hold your tiny, squirming bundle in your arms, glowing with post-birth exhaustion and joy. Then comes the question—should we do that extra genetic screen? “It’s just blood on paper,” they say, “It could save their life someday.” But what if it creates a shadow of health risks that never materialize? That’s the double-edged sword we’re slicing into today.
UK’s Bold 2030 Rollout
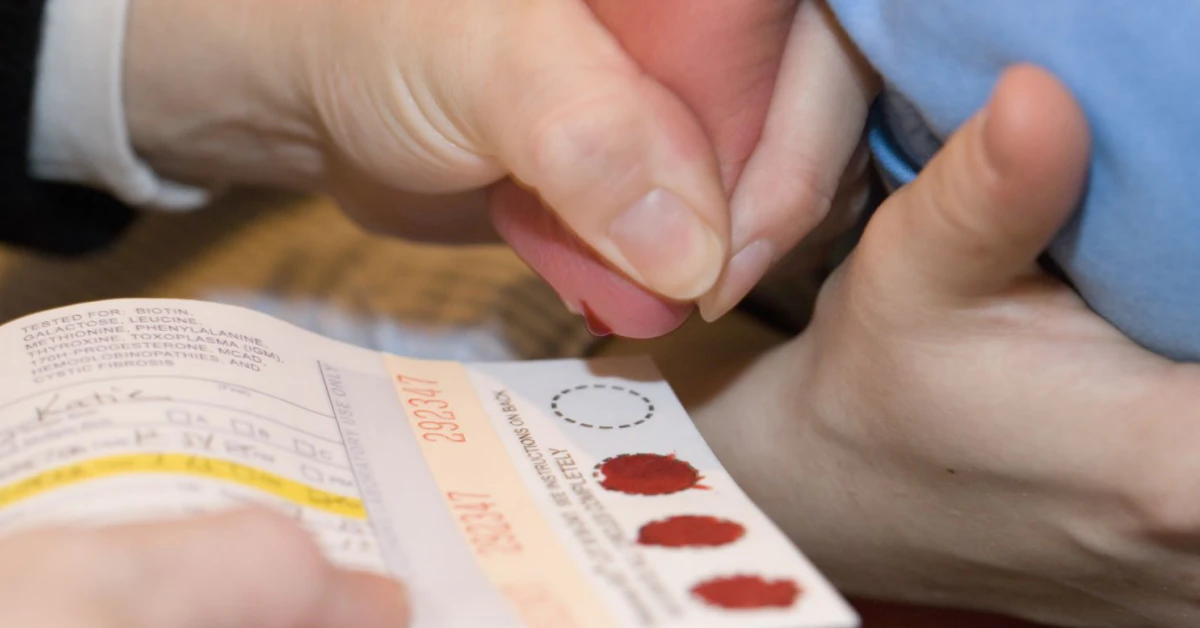
So, what’s the NHS changing? Right now, newborns in the UK get the classic heel-prick test—a few drops of blood on a spot, scanning for nine rare conditions. But the 2030 plan? That’s a genomic tsunami. They want to shift from the basics to something much deeper.
Here’s the vision: Every newborn’s DNA gets read cover-to-cover. Full genome sequencing isn’t just flipping through one chapter of health—it’s looking at all their biological stories. From metabolic red flags to cardiac canaries in the coal mine, this deluge of data could let doctors tackle problems before they even flicker into symptoms.
Now, is that fair? Some cheer the science—genetic detective work catching trouble early. Others shudder at the consequences: a generation of kids whose data might shift from medical tools to digital chains, dragging them into surveillance or discrimination later in life.
Benefits: When Early Is Everything
Okay, let’s talk about the bright side. If a condition is silent but dangerous—like PKU or CPT II deficiency—and a test catches it before disaster strikes, then medical science isn’t just improving care—it’s rewriting save lives from the inside out.
Here’s where it gets real: Sharon from Manchester learned her daughter had early signs of Congenital Hypothyroidism from the current heel-prick screen. Without treatment, the child might’ve faced hazy development and permanent challenges. Fast-forward six months, and the kid’s thriving, being screened early, juicing up the system a notch.
Or imagine Bobby, born in the US with Severe Combined Immunodeficiency (SCID). Under the heel-prick system, he might’ve never gotten the test till symptoms hit—too late. But because of a comprehensive supplemental test, his immune system got rescued in time. Stories like Bobby’s keep experts pumping their fists—they prove prevention can work when DNA gets the spotlight early.
That’s the crux: Tests like Fulgent Genetics’ panel analyze 258 genes, flagged for 200+ early-onset conditions. From blood and cardiac issues to pediatric cancer risks—there’s a roadmap. Problem is, reading DNA’s roadmap correctly isn’t always a walk in the park.
Risks: What If the Tests Get It Wrong?
Hazards pale against benefits? Not always. Here’s where the science starts cracking under pressure. Because while testing can flag a gene variant, it still mixes up what’s a blinking yellow light with a full-blown emergency siren.
Take bacterial infections closing in. Gene test shows red flags for SCID. Parents rush in for biopsies, scans, and treatments. Acute stress, minimal support for the parents, but it turns out the variant is benign. Or worse—inconclusive. No clear answer. Just swirling fears.
Harvard pediatricians point something unsettling—when NICUs get genetic results without medical clarity, some families unintentionally get pushed toward more aggressive courses because of the test, even if the child’s status is ambiguous. And that? That’s not just a data problem. It’s a human bias around genetic risks.
Here’s the science itself admitting stress: the AAP review found that NICU decisions are sometimes swayed by genome screens that offer “likely pathogenic” warnings—even when those annotations are still half-baked and invite fear instead of clarity.
FAQs Without the Clinical Glare
“Wait. So what’s in the test?”
Glad you asked. Let’s parse that flavor. The current UK heel-prick scan hunts for: phenylketonuria (PKU), SCID, cystic fibrosis, congenital hypothyroidism. That’s it—just nine conditions. But the new genomic vision? They’re gunning for the big leagues: hundreds of early-onset conditions.
Hearing loss—a hub of early childhood worry—has 18 comprehensive markers targeted in tests. According to CEN4GEN, blood, diabetes, immune conditions, and heart miswires get surfaced with gene hunts.
What about my baby’s genome privacy? Eh, that’s the gray zone. The cheapest private panels bottle up results to a pediatrician. NHS, however, plans to store those genes beyond—no official ntohs.yet. But ask yourself: who’s keeping that data safe? And once a flag is up, like a moderate hearing loss risk, will it ever come down?
False negatives—they happen? Of course. Even the stripped-down public tests can miss subtle variations. Think of it like walking into a kitchen and tasting only salt. You get part of the recipe, but doesn’t tell you what’s really cooking.
Here’s Your Parent’s Primer on Genetic Screening
Does every parent want the full genomic novel? Probably not. But part of the decision-making is feeling like you can choose. Let’s say your cousin, Karen, had her baby tested and found a variant that can be monitored. Then—over the next year—she caught symptoms easily with extra vigilance.
Gene tests are like that—a flashlight, not a GPS. But if you’re walking into the dark, you’ll reach for it.
So here’s what matters: how does the test influence care? Just discovering a variant shouldn’t get you anxious. The key is whether detection shifts reality. AAP’s research shows for certain conditions, diagnosis impact means faster care, but for others, it means endless testing limbo.
Still confused about what’s covered? Here’s how Fulgent Genetics chunks it:
| Category | Conditions Covered | Why It Matters |
|---|---|---|
| Metabolic Disorders | 148 genes, including PKU and CPT II | Early diet or enzyme therapies might prevent abuse to the central nervous system |
| Blood Conditions | 12 genes, like Thrombocytopenia spots | Catching clot issues early can reduce fatal bleeding episodes |
| Hearing Loss Flags | 18 on the front line: Connexin, Pendred syndrome | Kids with detected risks can get earlier audiograms, hearing aids |
This level of granularity shows how far beyond the heel-prick we’re pushing.
Emotional Drama: Prognosis vs. Real Life
You might think a genome read is just utility—you either use it or you don’t. But receiving the result, even ambiguous ones, can flip whole relationships. Imagine your baby in the NICU, gasping, fragile. A genetic test predicts lifelong disability risk. Even if the baby survives today—how do you feel knowing the twenty-five-year time bomb ticking inside their cells?
Parents walk a fine line here. Not all of them agree to extra screens. Some drop the bomb: “OMG, it’s DNA! What if it’s wrong?” while others clutch the results tightly—it’s a map. A baby’s bio—diabetes fully scratched out.
Katharine Press Callahan, a neonatologist out of Philly, warns—“We find changes we don’t know enough about to act. Some info buries possibilities instead of lifting.” And data security? Don’t even. Parents fear gene data slipping sideways, spooling out into insurance denials or creepy algorithm-targeted ads. Justifiable paranoia? Some think it’s not paranoid til it bites.
Steps to Deciding: Clarity First
So here’s what you do—before any pressure floods. Ask:
- “Will this screen change care in the next 6 months?” If lifetime unknowns, tread lightly.
- “Can we speak with a genetic counselor? Their job isn’t just science. They help you feel, not blind-sided by genetic jargon.
- “What’s the false positive rate here?” Know the game—and the stakes.
Also, remember: tests vary. Some cover the parental peace of mind zone. Others cast that damn wide net. You’ve got to know what you’re purchasing—because like vitamins or probiotics, some tests chime with high flinch rates.
If you’re in the UK and reading this prep for birth in a maternity ward? Say yes or no carefully. You’re not just opting into medicine—you may be volunteering your infant’s genome into decades of predictive research data. Is the collective win worth the private tradeoff? You decide.
Wrapping It Up: More Than Just Data
Genetic screening is distilling hope into a micro-array. It can catch heartblock alleles, metabolic storms, or bleeding liabilities with a genetic torch… but the shadows matter. If the tool doesn’t guide next steps, then the testing is just scattering fear.
You’ve heard both versions. My take? You don’t read genes like tarot cards. But with targeted tests and workable medicine, DNA profiles can sway outcomes.
We need genetic exploration, but called out for what it is—not a single “Okay, clear!” stamp on your health passport. It’s more like getting the Roadmap that includes traffic jams, detours, and some roads no one ever walked.
Got questions about testing? Want to share your family’s experience with gene flags? Feel free to jump in the comments. We’re in this together, babies and worried parents and all…
Raise a toast to the future, with all its uncertainties and wonders.
















Leave a Reply
You must be logged in to post a comment.