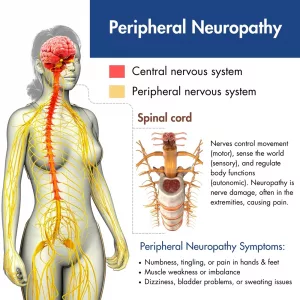
Fiber Mapping: Not Just a “First-World Problem” for Neuroscientists
Picture this: Scientists staring at scans like they’re trying to find Waldo in a sea of red and green pixels. Why? Because the brain’s nerve pathways—those pesky, spaghetti-strand-like structures—are notoriously hard to track. A recent Nature article called it the “holy grail” for diseases like glaucoma and multiple sclerosis. Yep, skin-deep imaging? Not good enough anymore.
Quick Wins vs. Underlying Struggles
Why are spinal pathways easier to image than peripheral fibers?
Your spine’s nerve roots? Thick and isolated like a well-lit highway junction. Peripheral nerves? Imagine photographing a needle in a haystack while it’s raining. At just 1-2mm, these little guys are buried under muscle, fat, and blood vessels. Back in 2010, MRI-tech journals (like this AJR study) hilariously labeled this as “the resolution paradox.” But jokes aside, partial-volume effects literally blur nerve vs. vein boundaries. Ever seen a blurry line between a hot dog and your finger? That’s what current scans risk here.
But what about 3D models in glaucoma research?
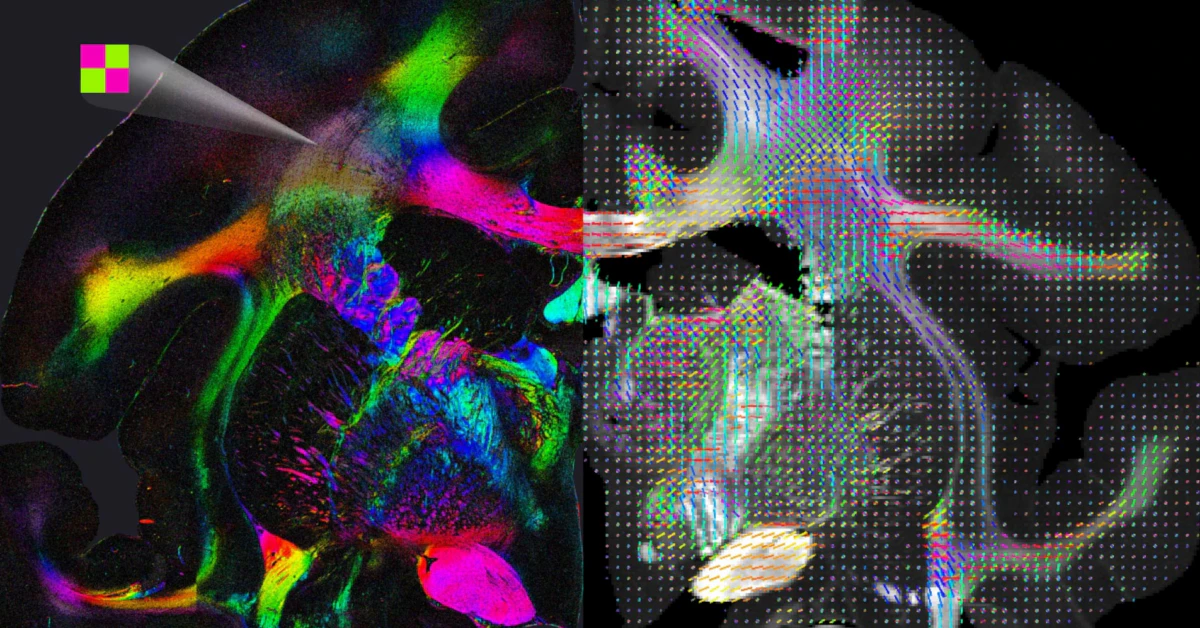
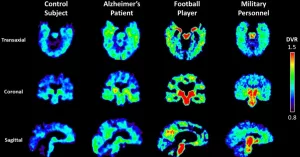
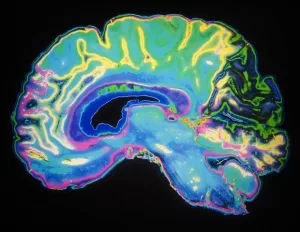
Flashback to 2003: MR Neurography’s debut let doctors and researchers “lift” the brain’s architecture into 3D. Why’s this important? Because glaucoma damages the optic fiber layer like dandruff fatiguing your hair—gently, then all at once. The HRT vs. OCT transition at UBC (glaucoma clinics switched entirely to OCT in 2012 due to accuracy) is a real-world example of how tools evolve when they must. But inspecting fibers crossing like tangled headphones? Still a no-go.
What Early Nerve Imaging Gets Back in Time
Why do some docs still use 1990s imaging tactics?
If my friend Sarah’s had a migraine for 8 months, her doctor might still order a visual field test—because it’s wildly cheaper than new tech. But this is like tracking traffic jams via pigeons and smoke signals. Historical context gives gravitas, though. Did you know? Fundus photography has been the standard since 1920! Although modern optics, it still resembles polaroids from your grandma’s kitchen.
CCM—Is it the Tesla of nerve imaging?
Corneal Confocal Microscopy (CCM) can non-invasively catch early nerve damage in kids with Type 1 diabetes. Cool, right? But imagine having an Instagram account but not knowing hashtags: that’s the barrier between CCM and widespread adoption. As StatPearls’ medical scholars pointed out, “lack of training stifles high-res microscopy’s golden age.” Which likely means a few workshop series away from becoming routine.
So, What’s in the Future Imaging Toolbox?
Are polarized light and scattering the perfect MRI match?
Clinics are starting to test hybrid tools (like combined polarimetric and scattering sensing) that read nervous tissue directionality. Think: Polarized sunglasses that still have windshields to see details at depth. Super-resolved imaging? Yep, it allows synapse close-ups, but you’re only scanning a tissue. It’s like checking symptoms with your magnifying glass instead of a holistic doctor’s eye.
| Modality | Strengths | Weaksauce |
|---|---|---|
| Optical Coherence (OCT) | Rapid, no radiation. Superior RNFL mapping | Struggles with hair-thin crossings |
| Diffusion Tensor Imaging (DTI) | 3D pathways, non-surgical plan | False tractography above 15° crossings |
| Confocal Microscopy (CCM) | In-vivo without a scalpel | Expensive. Interpretations vary 20% by clinic |
Help! My scan ordered more coffee. AI to the rescue?
We’ve all face palmed at Google Maps rerouting us through buildings. Similar manual tracking bugs plague early DTT scans—tossing out false negatives and phantom pathways. Enter 2025’s love-hate with machine learning (check MDPI’s CCM segmentations). Some AI systems now auto-complete nerve structures like we auto-complete lunch ideas on our phones. However, overhyped neural networks? Yeah, they can hallucinate connections faster than a fifth grader. The balance? (Forbes’ healthcare edition suggests it’s the “expert-in-the-loop” model where radiologists edit these tools instead of letting them go it alone.)
Are We Imaging a Solution or Getting a Makeup Exam?
Why do some docs still refuse the “build-a-brain” tools?
“This saves 5 minutes, but costs $12,000 per test.” Dr. Lawless, a neurologist from St. Mary’s Neurothritis Center, scratched the back of his gray hair while I overheard him at a pain clinic. That’s the legal reality—spanking new tech remains on paper until insurance covers it. Even Johns Hopkins’ surgical nerve repair (detailed here) sometimes still relies on old-school mapping. Cost barriers matter.
How to not “bite the AI bullet” wildly
Direct nerve repair sounds like a DIY project but requires exact imaging to even consider surgical fixes. If your camera shows an MRI pathway whispering into a path like a GPS that’s 90% precise, could surgery even be an option? That’s still debated. As PubMed puts it, even with upgraded DTT images of the brachial plexus, nerves can “dance out of view.”
Breaking Bad Fibers & Healthcare Tech That Isn’t Healed
Could AI rescue nerve imaging quality in remote clinics?
I live in a town 5 hours from the metro, and my optometrist still uses color fundus photos from 2003 tech. That’s where machine learning shines, though. Auto-detections have started bringing fancy medicine from California research centers into rural Australia and UK eye units, according to Frontiers in Pain Research (2025). By making scans easier to analyze, clinicians collect data without needing a 6-year fellowship in image theory.
But pump brakes—some “AI-ized” systems still send more false positives than OCRA certifications during peak flu season. Would an essay from 2018’s MRI experts toss a warning flag? Absolutely. Overfitting, training data from just one clinic’s database, and blurry scan-density thresholds keep CCGI reliability at “maybe I’ll use it but only as backup.”
The cost of precision? Sometimes… your two kidneys in eco.
K style: modern CCM tech adds tens of thousands to your testing bill (Cite: Cell Reports Methods, 2024). In a recent Philly case, a dad’s suspended insurance claim after a rushed CCM scan predicted diabetes-related nerve tears at 89% accuracy… but that 11% had him juggling three cryo clinics before settling. New imaging? Sounds fancy. But when applied in haste? Sometimes just fancy time spent without healing.
Humans, Hardware, and the Nerd Dust We Breathe
Can microscopes become strong enough to fix wall-to-wall nerves?
Today’s mesoscale imaging tech—this decade’s Picasso—is still getting stuck on multiple color axes, targeting fluorescent dyes. But new Wiley research (2025) suggests hybrid setups where you “color by wavelength” and “illuminate by scattering.” Imagine: 3D imaging that evolves hour by hour to regrowth and injuries—without relying on stains or intimating hardware. That’s next year’s rooftop vision.
But wait—does MRI still **** its pants around lungs/neck areas?
John Wiley taught us the neck’s MRI field inhomogeneities are not just problems—they’re showstoppers (link: current AJR note). Imagine trying to photograph your child’s first edge on a screen while the TV glitches between chromatic aberrations. Sucks. By the time the software tries to automap the above/phrenic accelerations, strands already cross like off-brand IKEA instructions. But are we tackling it? Surprisingly—yes! MRN’s “field mapping during breath holds” let someone park their organs in one spot temporarily. Futuristic hospital bluffing material, this.
Final Verdict: Head or Heart in Neuroimaging’s Future?
This isn’t just about snazzier soundbites on Neurotech podcast episodes. It’s real: a child feeling numbness at 14 could equal diabetes warning. A 62-year-old migraine sufferer might uncover a fibrosis trend before becoming blind. Does that make the heady technical side worth it?
I vote yes. But let’s be real—the “what next” chapter hangs in clinics’ willingness to teach old docs new tricks and manufacturers cutting the absurd costs of next-gen microscopes. Otherwise, we just have a showcase in Switzerland and nothing in Grand Rapids, where the scanners still look like Lancet-predicted 2018 interface tech. And trust me, we can’t afford to block that bridge.
Got questions? Struggling to understand why your doc uses one modality but not another? Shoot me a comment (or even a carrier pigeon—we’ll decode it together ;). Because if nerve pathways are finally untangling their histories, shouldn’t we be part of the conversation too?













Leave a Reply
You must be logged in to post a comment.