Let’s Start With the Real Stuff
It’s exhausting, isn’t it? You get through chemo, stem cell transplants, maybe a dozen pill bottles on your nightstand… only to hear the words “your myeloma is back.” Ugh, again?! If any of this sounds close to home, you’re absolutely not alone. Multiple myeloma loves a comeback, but so do people living with myeloma—fighting for time, quality, and hope.
But here’s where things get interesting (and maybe a bit hopeful): the universe of cell therapies. You’ve probably heard about “CAR T-cell therapy” in those support groups or late-night Google deep-dives. These days, two names pop up on repeat—Abecma and Carvykti. But every time you ask for the honest, side-by-side scoop—Abecma vs Carvykti—there’s just more jargon. So, what’s the real deal for people like us, not just lab data?
This CAR Road Has Two Lanes
First off, these are both FDA-approved CAR T options for relapsed or refractory multiple myeloma. Abecma (idecabtagene vicleucel) led the way, securing its first FDA nod back in March 2021 (Oh, what a year, right?). Then came Carvykti (ciltacabtagene autoleucel), sweeping in with its own approval in February 2022. Lately, both have gotten a big green light to be used much earlier in treatment, not just after you’ve “failed” all the typical stuff.
For anyone who’s been counting down the days and lines of treatment (you know that game), the Abecma approval date and its evolution matter—a lot. It’s not just some box on a doctor’s form; it could mean stepping out of that endless relay of chemo and into a chance for a longer, treatment-free break.
So, How Do These CAR Ts Really Work?
Here’s my friend-to-friend version: CAR T-cell therapy is when scientists take your own T-cells (those hardworking immune soldiers), genetically tweak them to find and attack BCMA markers on myeloma cells, then send them back in… like setting your immune system on a special ops mission. Seriously, it sounds wild, but it’s poetically simple—”Hey body, here’s how you find the bad guys hiding out.”
Both Abecma and Carvykti do this. But, when you zoom in, Carvykti’s T-cells are like double agents—they target two different spots (epitopes) on BCMA, while Abecma goes after one. Scientists geek out over this because it might make a difference in how deeply and/or how long the CAR T-cells can keep those sneaky myeloma cells away. According to research on Carvykti’s FDA approval, this dual targeting could explain why some studies point to higher response rates for Carvykti, but let’s not get ahead of ourselves…
Showdown: Abecma vs Carvykti (And What Do The Numbers Mean Anyway?)
All right, let’s break this down like two old friends over coffee (OK, maybe herbal tea with all those anti-nausea drugs): What does the data actually say about Abecma vs Carvykti?
First thing’s first—there’s no single “winner.” Your perfect match depends on your unique story, age, treatment history, all that jazz. But there are meaningful differences.
Response Rates and Remission—Who Packs A Bigger Punch?
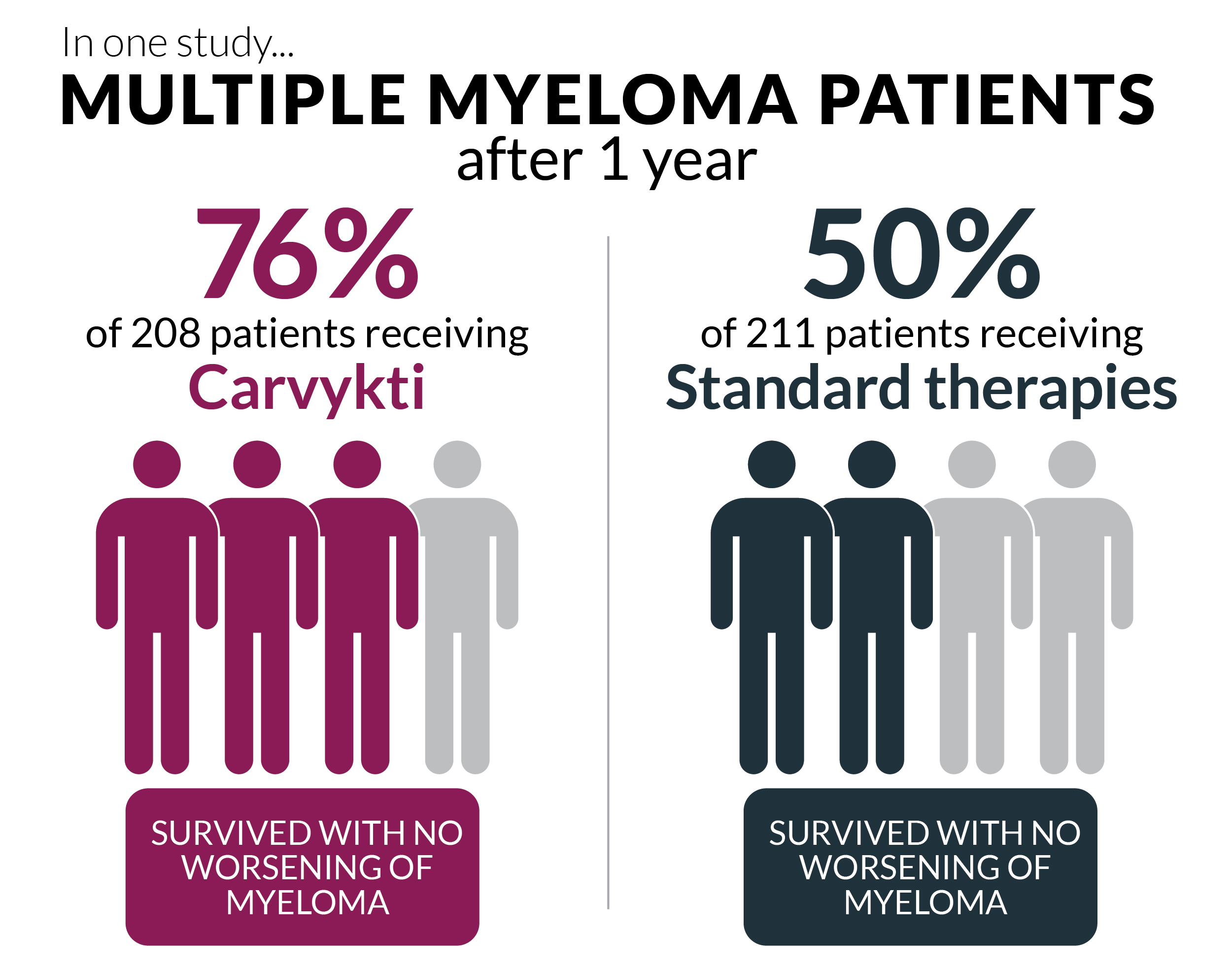
Studies and real-world stories are piling up, and a pattern’s emerging. Carvykti is edging ahead in response rates—like, a lot. In pivotal trials, Carvykti showed an overall response rate (ORR) up to 98%. For Abecma, it’s closer to 73–80%, still stellar when compared to old-school regimens but not quite as eye-popping as Carvykti. And complete remission? Carvykti clocks in up to 83% in some studies, blowing past Abecma’s 33%–39% range.
It actually reminds me of Lisa, a woman in my local support group who’d relapsed four times. Two years ago, she rolled the dice on Carvykti as part of a trial—not only did her numbers plummet, but she got almost three years without needing ongoing treatment. “I forgot what it felt like to live between infusions,” she told us, and geeze, isn’t that the whole point?
Table: Efficacy Features of Abecma vs Carvykti
| Feature | Abecma | Carvykti |
|---|---|---|
| Overall Response Rate | 73–80% | 98% |
| Complete Remission Rate | 33–39% | up to 83% |
| Median Progression-Free Survival | ~8–13 months | up to 35 months |
(Source: CARTITUDE-1, KarMMa, matching-adjusted indirect comparisons—see full Carvykti research and ABECMA FDA approval history)
But Wait—What About The Side Effects?
Here’s where things get real… because anyone who’s survived a round of myeloma drugs knows the side effects can be the actual worst part. Both therapies can cause what docs call cytokine release syndrome (CRS)—fever, chills, sometimes feeling like you got hit by a truck—and neurotoxicity (think confusion, headaches).
According to a retrospective analysis, Carvykti sometimes brings more severe CRS and neurotoxicity, mostly handled with careful monitoring and supportive care. In plain English? Both therapies can put you through a rough couple of days in the hospital, but there’s a well-dialed-in care plan (and reassuringly, most folks recover well). Some patients might face a higher infection risk or delayed nerve issues, though, so it isn’t a total “get out of jail free” card on side effects.
One guy at my center—Mark, early 60s—had Abecma, got chills and a fever so high he joked he could “brew tea with his face,” but bounced back quickly and felt more like himself within a week. Stories like his can help you weigh “worth it?” in your own life… which, by the way, is totally your call.
Another Quick Table: Safety Side-by-Side
| Side Effect | Abecma | Carvykti |
|---|---|---|
| CRS—Any Grade | ~84% | ~75% |
| Severe CRS (Grade 3+) | 2–4% | 5–7% |
| Neurotoxicity—Any Grade | ~22% | ~14% |
| Severe Neurotoxicity | 4% | 4–6% |
| Secondary Malignancies | Rare | Trend toward higher, but rare |
(Source: HealthTree patient analysis)
When Can You Actually Get These Treatments?
Here’s the other big piece—good news if you’re tired of being told you’re “not eligible… yet.” Thanks to fast-moving approvals this year, Carvykti is now available after just one prior line of therapy (if you’re also refractory to lenalidomide), while Abecma is available after two or more prior treatments (Abecma approval date and updated FDA indication tell the story). So you don’t necessarily have to bounce from chemo to transplant to more chemo on an endless hamster wheel before someone says, “Let’s try CAR T.”
If you want to nerd out on the timelines (like I did), check out the ABECMA FDA approval history. It shows just how fast the world of myeloma treatment is moving. Five years ago, even the first attempts at CAR T felt hush-hush and experimental. Now? These are real, mainstream options in major cancer centers.
How Do You Choose? (And Is It Really A Choice?)
Okay, so… which is right? The unsatisfying answer is: it depends. Every story is personal. Your age, health, what you’ve already tried—each factor could nudge your doc toward one or the other.
Sometimes, access or insurance or even how close you live to a major center can make the biggest difference. One patient I know, Maya, wound up with Abecma because it was available sooner—she literally said, “The calendar picked for me.” But now with Carvykti approved earlier, those logistics are changing at light speed.
One practical tip: Ask your team which cell therapy center they use. Some have more experience with one product or may know about trial slots for the other. Never hurts to put it plainly: “If I needed CAR T, which would I get here?” (And yes, you’re allowed to keep asking…)
What’s Next? Are We Done, Or Is This Just The Beginning?
If there’s one thing I’ve learned as a myeloma traveler: the journey never stays still. CAR T-cell therapy—both Abecma and Carvykti—is still evolving. We’re seeing more trials, combo therapies, and maybe… just maybe… a future where “cure” is said out loud. There’s even data from China showing Carvykti’s long-term results, suggesting around 30% of patients could remain in response for six years or more (real-world long-term study). That’s… well, kind of wild, isn’t it?
Whether you’re team Abecma, leaning Carvykti, or still weighing your options, give yourself space to be a little messy, a little confused—just like the rest of us. There’s nothing neat about cancer, but the fact that there are now several roads to try? That’s massive.
Your Turn: Questions, Hope, Next Steps
So, what’s swirling through your head right now? Are you thinking, “Is CAR T right for me?” or “How soon can I do this?” Maybe even, “How do I make sense of these risks and numbers?” These are all good, honest questions.
If you’re up for it, bring up the Abecma approval date or just straight-up ask for the ABECMA FDA approval history at your next appointment—that way you’ll be armed with recent info. Remember: your experience matters just as much as the data.
Have you ever noticed how the biggest leaps in treatment don’t always come from the scientists alone, but from people like you—asking hard questions, saying “enough” to the old routines, demanding more? That’s how Abecma and Carvykti made it past clinical trials and into real lives.
Wrapping Up: Keep Going, Stay Curious, You’ve Got This
We covered a bunch here, right? From approvals to response rates, chemo nightmares to sunlit stretches of remission. Whether you’re planning, coping, or just plain tired and looking for hope, know this: Abecma vs Carvykti is more than a science debate—it’s about options, empowerment, and grabbing a slice of normalcy back from myeloma.
Keep being your own advocate. Keep asking. Stay connected with your care team and with other folks traveling the same road. Because one day soon, you might be the one telling a newly diagnosed friend, “CAR T changed my life.” And wouldn’t that be something?
You deserve answers, support, and—if it’s possible—a stretch of time to just live again. Keep fighting for that. And hey, if you made it to the end of this article, you’re already stronger than you know.

















Leave a Reply
You must be logged in to post a comment.