Quick Answer Overview
Alright, let’s cut to the chase. Gomekli (generic name mirdametinib) is an oral, small‑molecule MEK inhibitor that the FDA approved in February 2025 for adults and children ≥ 2 years old who live with neurofibromatosis type 1‑plexiform neurofibromas (NF1‑PN). In plain English, it’s a pill that helps slow down the growth of those stubborn tumors that can’t be surgically removed.
The drug comes as 1 mg or 2 mg capsules, and a 1 mg tablet that can be turned into a liquid suspension. The standard regimen is 2 mg/m² taken twice daily for 21 days followed by a 7‑day break each 28‑day cycle.
If you’re wondering whether this could be the right option for you or a loved one, keep reading—there’s a lot more to unpack, and I promise to keep the jargon to a minimum.
Why It Matters
NF1‑PN is a rare genetic disorder where benign tumors grow along nerves, often causing pain, disfigurement, and—worriedly—risk of turning malignant. Before Gomekli, treatment options were basically “watch and wait” or invasive surgery, which isn’t always possible because the tumors snake through nerves like a tangled ball of yarn.
Enter the Gomekli approval story: SpringWorks Therapeutics ran the pivotal Phase 2b ReNeu trial (NCT03962543). The FDA gave the green light on Feb 11 2025 after the study showed meaningful tumor shrinkage and an acceptable safety profile. That moment felt like a sunrise for the NF1 community—finally, a medicine specifically built for this condition.
Here’s the kicker: In the adult cohort, 41 % of participants saw their plexiform neurofibromas shrink by at least 20 %, and 15 of those enjoyed reductions of more than 50 %. For kids and teens, the numbers were equally encouraging—over half of the children in the trial achieved that same 20 % shrinkage, with a median response time of just under eight months. Those statistics aren’t just numbers; they’re real people getting a little more room to breathe, move, and live without the constant shadow of tumor‑related worries.
How It Works
Think of the MEK pathway as a highway that tells cells when to grow and divide. In NF1‑PN, that highway is stuck on “go,” leading to uncontrolled tumor growth. Gomekli acts like a traffic cop, blocking the MEK1 and MEK2 proteins (the “stop lights”) so the cells get the message to slow down.
From a pharmacology standpoint, the drug is a multikinase inhibitor that binds directly to MEK enzymes, halting the downstream ERK signaling cascade. The result? Tumor cells stop proliferating, and many begin to shrink. The approved dosage—2 mg/m² twice daily—was derived from careful dose‑finding studies that balanced efficacy with manageable side effects.
For those who prefer a liquid form, the 1 mg tablet can be dissolved in water, making it easier for younger kids or anyone who has trouble swallowing capsules. That flexibility helped the trial enroll a broad age range, from toddlers to adults.
Safety Profile Details
No medication is completely free of risk, and Gomekli is no exception. The good news is that most side effects are predictable and can be managed with routine monitoring.
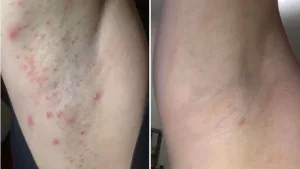
Common (≥ 10 %) side effects include:
- Rash (often itchy or red)
- Diarrhea, nausea, or vomiting
- Stomach discomfort
- Muscle, joint, or bone pain
- Fatigue and headache
Serious concerns that need close watching:
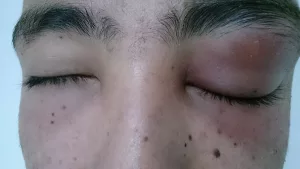
- Ocular toxicity – blurred vision or eye pain; baseline eye exams and periodic checks are mandatory.
- Cardiac effects – decreased left ventricular ejection fraction (LVEF). Baseline echocardiograms and follow‑up imaging help catch any dip early.
- Hematologic changes – low white blood cell counts and elevated creatine phosphokinase (CPK). Regular blood work is part of the safety protocol.
Managing these issues is part of the treatment journey. If a rash appears, moisturizers and antihistamines often do the trick. Diarrhea can be tackled with over‑the‑counter agents like loperamide, and many clinicians recommend taking the medication with food to soften stomach upset.
One practical tip I’ve heard from families: use the “off‑week” as a window to reset. For example, a caregiver shared that her son experienced headaches during the break, so they gave a mild pain reliever a day or two after the off‑week began and the symptoms eased. Simple adjustments like that can make a big difference in daily comfort.
For a deep dive into every possible adverse reaction, you can check the Gomekli side effects guide we’ve put together.
Who Should Consider
If you or someone you love has been diagnosed with NF1‑PN and the tumors are causing pain, functional problems, or are simply too big to ignore, you’re probably a candidate for Gomekli. Here’s a quick eligibility checklist:
- Confirmed NF1‑PN diagnosis (MRI‑verified plexiform neurofibromas).
- Age ≥ 2 years (the drug isn’t cleared for infants).
- Symptoms that affect quality of life or the tumor cannot be fully resected.
- Willingness to undergo baseline eye exams, cardiac echo, and routine blood work.
Before starting, your doctor will want a baseline panel that includes:
- Comprehensive ophthalmic exam.
- Echocardiogram to assess heart function.
- Complete blood count (CBC) and chemistry panel.
Insurance coverage can feel like navigating a maze, but many patients qualify for SpringWorks’ co‑pay assistance program. The typical wholesale price hovers around several thousand dollars a month, but manufacturer assistance and some private insurers can bring that number down considerably. For a more granular look at the price breakdown, see our Gomekli cost page.
Bottom Line Summary
To wrap things up, Gomekli represents a major breakthrough for anyone living with NF1‑PN. It offers a realistic chance of tumor shrinkage, comes in a convenient oral form, and—when paired with diligent monitoring—has a safety profile you can manage with the right support team.
That said, it’s not a “set‑and‑forget” pill. Regular eye checks, heart ultrasounds, and blood tests are non‑negotiable, and side effects, while often mild, do require open communication with your healthcare provider.
If you think Gomekli could be a game‑changer for you or a family member, the first step is to talk to a physician who specializes in NF1‑PN. Ask about the ReNeu trial data, request the FDA label, and explore the patient‑finder tool on the official GOMEKLI website to locate a specialist near you.
Remember, you’re not alone on this path. Communities of patients, caregivers, and advocates are sharing stories daily—some see 57 % tumor reduction after a year, others learn to navigate the occasional rash with a simple cream. Sharing experiences makes the journey less daunting, so don’t hesitate to reach out, ask questions, and stay hopeful.
What’s your next move? If you have more questions about Gomekli, feel free to explore the resources linked throughout this article or start a conversation with your doctor today. You deserve clear, compassionate information—and we’re here to help you find it.

















Leave a Reply
You must be logged in to post a comment.