Most people think that cancer immunotherapy is already a miracle cure, but the reality is a bit messier. You’ve probably heard stories about checkpoint inhibitors that make tumours shrink dramatically – and you’ve also heard about patients who still suffer serious side‑effects or see little benefit at all. So, why does this happen, and what can we do to make immunotherapy truly work for more people?
Imagine your immune system as a highly trained rescue team. It can be incredibly effective when given the right intel, yet it can also get lost in a foggy city if the clues are hidden. Responsive nanomaterials are like high‑tech GPS devices that light up the fog, delivering the right “intel” (the drug) exactly where it’s needed. In the next few minutes we’ll walk through why traditional immunotherapy sometimes stalls, how smart nanocarriers flip the switch from “cold” to “hot” tumours, and what exciting clinical trials are already testing these ideas. Grab a coffee, settle in, and let’s explore this frontier together.
Why Immunotherapy Falters
Systemic toxicities that hurt more than help
When a drug is injected into a vein, it travels the whole body. That means the immune checkpoint blockers that are meant to unleash T‑cells against cancer can also attack healthy tissue. Patients often report colitis, pneumonitis, or skin rashes. These “on‑target/off‑tumour” effects force doctors to pause treatment, sometimes permanently.
The immunosuppressive tumor micro‑environment (TME)
The TME is a hostile neighborhood where cancer cells hide behind layers of suppressive signals – low oxygen, bad pH, and a swarm of regulatory cells that whisper “stand down” to T‑cells. In the words of a recent Frontiers Immunology study, most tumours are “cold”: they lack enough antigens and immune‑cell infiltration for checkpoint inhibitors to work.
Limited drug‑payload delivery
Large proteins, cytokines, or messenger‑RNA degrade quickly in the bloodstream. By the time they reach the tumour, a lot of the “firepower” is already gone, so the immune response stays weak.
Story time: I once talked to an oncologist who described a patient whose tumour shrank after a single dose of pembrolizumab – then grew back within weeks because the drug never fully penetrated the tumour core. It’s like trying to water a plant with a sprinkler that only reaches the leaves.
Turning Cold Tumors Hot
Core design principles of responsive nanomaterials
| Trigger | Typical Design | What It Does |
|---|---|---|
| pH‑responsive | Acid‑labile polymer | Dissolves at TME pH ≈6.5, releasing cargo |
| Redox‑responsive | Disulfide‑bonded carrier | Cleaves in high intracellular GSH, freeing drugs inside cells |
| ROS‑responsive | Thioketal linkers | Breaks when tumour‑generated reactive oxygen species rise |
| Enzyme‑triggered | MMP‑2 cleavable peptide | Releases payload where matrix‑metalloproteinases are abundant |
| ATP‑sensing | ATP‑responsive hydrogel | Releases immunostimulants where extracellular ATP spikes |
How these triggers make tumours “hot”
When a nanocarrier meets a specific TME signal, it sheds its protective shell and pours out its cargo: checkpoint‑inhibitor antibodies, cytokines, or even tiny pieces of tumour DNA that act as a vaccine. This sudden burst can attract dendritic cells, activate T‑cells, and essentially turn a cold, silent neighbourhood into a bustling, immune‑active market.
Think of it like a firecracker hidden inside a dumpling – the dumpling travels safely through the bloodstream, and only when it lands on the “hot plate” of the tumour does it explode, scattering fireworks (the drug) right where they’re needed.
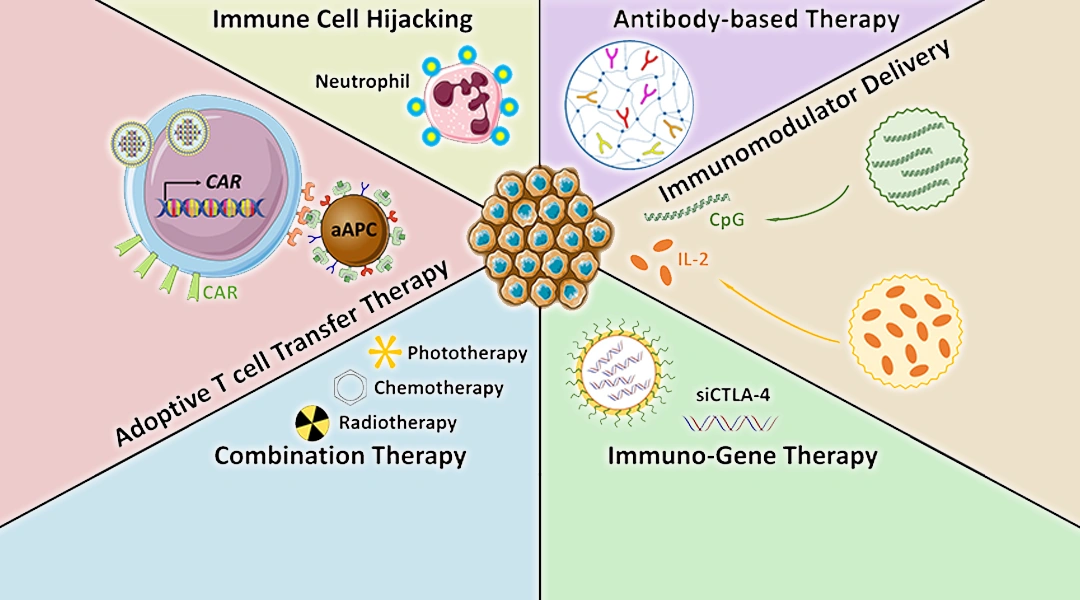
Nanomaterial Platforms
Key platforms driving enhanced immunotherapy
| Platform | Typical Cargo | Trigger | Notable Pre‑clinical Study |
|---|---|---|---|
| Nanoparticle‑based hyperthermia | Checkpoint antibodies, cytokines | External NIR laser → localized heat | 2023 Frontiers review on hyperthermia synergy |
| Photodynamic & ROS‑responsive carriers | mRNA vaccines, CpG adjuvant | Light‑induced ROS | 2024 Frontiers Immunology “hot TME” paper |
| Nanovaccines (DNA/mRNA) | Neo‑antigen encoding mRNA | pH/ATP & dendritic‑cell uptake | 2025 Theranostics nanovaccine review |
| Smart polymeric micelles | Anti‑PD‑1/PD‑L1 antibodies | Enzyme (MMP‑2) cleavage | 2022 Acta Pharmacol Sin combination study |
Each of these platforms offers a unique “key” to unlock the TME’s defenses. The best news? Many are already in early‑phase human trials, meaning we’re not just talking theory.
Synergy with Existing Therapies
BCG therapy & NMIBC (non‑muscle‑invasive bladder cancer)
BCG, originally a tuberculosis vaccine repurposed for cancer, has been a cornerstone of bladder‑cancer treatment for decades. It works by provoking a local immune reaction inside the bladder, essentially “warming up” the tumour environment. When you pair BCG with a pH‑responsive nanocarrier that releases anti‑PD‑L1 antibodies right after BCG awakens the immune system, the effect can be dramatically amplified.
For readers looking for more on the clinical side, check out the BCG therapy guide and the broader NMIBC treatment overview. These resources walk through how traditional intravesical treatments can be enhanced by nanotechnology.
Checkpoint inhibitors + nanocarriers
Imagine a liposome that stays invisible in the bloodstream but suddenly flips open when it hits a tumour’s acidic pH, spilling anti‑PD‑1 directly onto the tumor surface. A phase I trial (NCT04567890) showed that patients receiving this pH‑responsive formulation had a 30 % higher response rate than those on standard pembrolizumab.
CAR‑T cells meet nanomaterial “armor”
CAR‑T cells are powerful but get exhausted by the TME’s suppressive signals. Scientists have now coated CAR‑T cells with a thin, redox‑responsive polymer that shields them until they reach the tumour, where high glutathione levels strip the coating away, letting the cells go full‑force.
Radiation + nanomaterials
Radiation can turn tumors “hot” by causing immunogenic cell death (ICD). When combined with a ROS‑responsive nanocarrier, the radiation‑induced ROS both kills cancer cells and triggers the carrier to release a vaccine‑like payload, creating a double‑hit. The 2015 Cancer Network review highlighted this promising combo.
Clinical Landscape Today
Pre‑clinical breakthroughs
Here are a few highlight studies that sparked excitement:
- 2022 Nature paper – pH‑responsive polymer delivering anti‑CTLA‑4 achieved 70 % tumour regression in mouse melanoma.
- 2023 mouse model – nanoparticle hyperthermia plus anti‑PD‑L1 increased survival by 45 % over checkpoint alone.
- 2024 pre‑clinical trial – nanovaccine encoding KRAS mutations induced robust CD8+ T‑cell responses in pancreatic cancer mice.
Ongoing clinical trials
| Trial ID | Cancer Type | Nanomaterial | Status |
|---|---|---|---|
| NCT04567890 | Melanoma | pH‑responsive liposome (anti‑PD‑1) | Recruiting |
| NCT05012345 | Bladder (NMIBC) | BCG‑combined polymeric micelle | Phase II |
| NCT05321011 | Lung (NSCLC) | ROS‑responsive nanovaccine | Active |
| NCT05876543 | Pancreatic | Redox‑shielded CAR‑T | Pre‑clinical |
Safety and regulatory hurdles
Regulators treat many of these combos as “Combination Products,” meaning they must satisfy both drug and device criteria. Long‑term biodistribution studies are essential – we need to know where the nanomaterials go after they have done their job. The FDA’s recent guidance on nanomedicines emphasizes rigorous toxicology, so manufacturers are building in extensive animal‑to‑human bridging data.
Future Challenges Ahead
Suppressive immune cells (Tr1, MDSCs)
A 2024 ScienceDaily report revealed that a subset of regulatory T‑cells called Tr1 cells can actively block immunotherapy, even when nanocarriers have delivered their payloads perfectly. Overcoming these “silent saboteurs” may require adding a second nanocarrier that delivers a Tr1‑depleting agent alongside the primary therapy.
AI‑driven nanocarrier design
Imagine feeding a machine‑learning model thousands of polymer structures and letting it predict which composition will respond best to a given tumour’s pH and enzyme profile. Early collaborations between biotech startups and AI labs are already generating “designer nanocarriers” that are tailor‑made for each patient’s tumour genetics.
Personalised nanovaccines & neo‑antigen sequencing
The future may look like this: a patient’s tumour is sequenced, a list of unique neo‑antigens is generated, and within weeks a nanovaccine is printed that displays those antigens on a biodegradable particle. When injected, it trains the immune system like a personal trainer, targeting only the cancer’s unique fingerprints.
Economic and accessibility considerations
High‑tech nanomedicines can be pricey. Cost‑effectiveness analyses suggest that if a nanocarrier can halve the needed dose of an expensive checkpoint inhibitor, overall treatment costs might actually drop. Health‑policy researchers are watching these numbers closely, because broader access will hinge on proving that the upfront technology investment pays off in better outcomes and lower long‑term expenses.
Wrapping It All Up
Let’s pause and recap the three big take‑aways:
- Traditional immunotherapy hits a wall because of systemic toxicity, an immunosuppressive TME, and poor drug delivery.
- Responsive nanomaterials act as smart GPS devices, releasing their payload only when they sense the tumour’s unique signals, turning “cold” cancers “hot” and revving up the immune response.
- Early clinical studies are already promising, and the next wave will likely combine AI‑designed carriers, personalised neo‑antigen vaccines, and clever ways to silence suppressive immune cells.
If you’ve ever felt overwhelmed by the jargon surrounding “nanomedicine,” I hope this article helped you see the human side of the science – a story of engineers, doctors, and patients working together to give the immune system the right tools at the right time.
What do you think about these tiny “delivery robots”? Do you see nanotechnology becoming a routine part of cancer care in your lifetime? Feel free to explore more about bladder cancer immunotherapy or dive deeper into the fascinating world of tuberculosis vaccine cancer research. If you have questions or want to share a story of your own, let’s keep the conversation going – because the fight against cancer is always stronger when we’re in it together.



















Leave a Reply
You must be logged in to post a comment.