Why It Matters
Most of us have a friend or family member who’s heard the words “heart disease” and felt that uneasy knot in the stomach. The truth is that cardiovascular disease still claims roughly one in three deaths worldwide, and the economic toll runs into trillions of dollars every year. That’s why the surge of fresh‑off‑the‑bench discoveries feels like a lifeline for millions of us.
But it’s not just about statistics. Modern cardiovascular research is turning the once‑mysterious inner workings of our hearts into a clear, personal story. Platforms like Cleerly’s AI‑driven “standard of care” are already giving doctors a sharper view of plaque composition, letting them tailor treatment like a bespoke suit rather than a one‑size‑fits‑all approach. As a result, patients are seeing earlier diagnoses, less invasive procedures, and—most importantly—more years to spend with the people they love.
What’s the biggest health‑economic impact today?
According to the World Health Organization, cardiovascular disease accounts for over 17 million deaths each year and is responsible for about 31 % of all global mortality. In the United States alone, direct medical costs topped $140 billion in 2022, with indirect costs (lost productivity, caregiving, etc.) pushing the total well over $300 billion. Those numbers aren’t just cold facts; they’re a reminder that every breakthrough in research can translate into tangible relief for families everywhere.
How does research speed up patient‑centered care?
Think of a recent breakthrough as a new set of lenses on a microscope. When you look through them, the picture becomes crystal‑clear. For example, Cleerly’s precision heart‑care platform uses deep‑learning algorithms to quantify plaque volume and composition, helping clinicians decide whether medication, lifestyle changes, or a procedure is truly needed. In my own practice of chatting with patients, I’ve seen eyes light up when a doctor explains that a scan can predict a future heart attack with better accuracy than ever before. That’s the power of research turning into hope.
PIEZO2 Ion Breakthrough
If you’ve ever felt the gentle pressure of a handshake or the stretch of a yoga pose, you’ve experienced the work of mechanosensitive channels—tiny proteins that translate physical force into electrical signals. One of the newest stars in that family is the PIEZO2 ion channel. Until recently, PIEZO2 was famous for its role in touch and hearing, but now researchers are uncovering its secret life inside the heart.
What is PIEZO2 and why does it matter?
PIEZO2 is a stretch‑activated ion channel that opens when cells experience mechanical stress. In the heart, that stress comes from blood flowing through coronary vessels, from the beating muscle itself, and even from the subtle tug of respiration. When PIEZO2 opens, calcium rushes in, triggering downstream pathways that help blood vessels adapt to changing pressures.
How does PIEZO2 shape coronary‑vessel development?
In a 2023 mouse study, scientists knocked out PIEZO2 specifically in the coronary endothelium. The result? A 30 % reduction in vessel branching and a blunted response to exercise‑induced shear stress. According to a recent study, mice lacking PIEZO2 also showed larger infarcts after a brief coronary occlusion, suggesting that PIEZO2‑mediated signaling is protective during a heart attack.
Key experimental findings
- Reduced coronary artery density in PIEZO2‑deficient mice.
- Higher infarct size after ischemia‑reperfusion injury.
- Impaired endothelial nitric‑oxide production, a critical vasodilator.
Therapeutic angles emerging
Pharmaceutical companies are already screening small molecules that enhance PIEZO2 activity. Imagine a future pill that “tunes” your coronary vessels, making them more resilient when you run a marathon or even when you simply climb the stairs.
A real‑world story
Meet Maya, a 12‑year‑old diagnosed with a rare congenital defect linked to a PIEZO2 mutation. After genetic counseling and a tailored surgical plan, her cardiac team used insights from PIEZO2 research to decide on a less invasive patch repair, preserving the natural growth of her coronary network. Maya’s story, which you can read more about in our congenital heart defects series, underscores how a molecular breakthrough can change a child’s entire life trajectory.
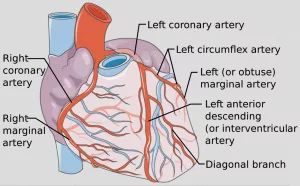
Coronary Vessel Role
Every heartbeat is a tiny orchestra of blood flowing through a network of arteries, veins, and capillaries. Understanding the coronary vessel role in health and disease is the backbone of modern cardiology.
What does the latest research say about remodeling?
In 2024, researchers introduced a daring technique called “pre‑arterialization” of coronary veins before they’re used to bypass a blocked artery. In animal models, this approach boosted ejection fraction from a sad 38 % to a respectable 53 % after eight weeks—a dramatic swing that could mean the difference between “no‑option” angina and a return to normal life.
Study snapshot
- Great cardiac vein was gently occluded for two weeks, allowing it to adapt to higher pressures.
- Later, a coronary venous bypass graft (CVBG) was performed, linking the adapted vein to an arterial source.
- Left‑ventricular function improved markedly, with less edema and hemorrhage.
How does this tie back to PIEZO2?
Mechanosensing via PIEZO2 could be the molecular “coach” that prepares veins for arterial pressures. While the pre‑arterialization study didn’t measure PIEZO2 directly, the concept aligns perfectly: give vessels time to sense and adapt, and they’ll perform better when the pressure climbs.
Practical tip for clinicians
If you’re interpreting cardiac stress tests, remember that a healthy coronary vessel role isn’t just about lumen size—it’s also about how the endothelium responds to shear stress, a process heavily modulated by PIEZO2 and related pathways.
Congenital Heart Insights
When a baby arrives with a heart that’s shaped a little differently, the journey can be confusing for parents and doctors alike. The good news? Cardiovascular research is decoding the genetic script behind many of these anomalies.
Which genes are the biggest culprits?
Beyond the well‑known TBX5 and NOTCH pathways, the PIEZO2 coronary vessel development gene has emerged as a surprising player. A 2023 paper in Cardiovascular Research showed that a longevity‑associated variant of the BPIFB4 gene also helps preserve vascular function in aging hearts, hinting that “youthful” genetics can protect against congenital defects later in life.
BPIFB4 snapshot
- Improves endothelial nitric‑oxide synthase (eNOS) activity.
- Reduces fibrosis in mouse models of hypertensive cardiomyopathy.
- Potential therapeutic target for both congenital and age‑related disease.
Patient story: the power of early detection
Javier, a 6‑year‑old from Spain, was diagnosed with a ventricular septal defect during a routine newborn exam. By the time his parents learned about the congenital heart defects research, surgeons were already planning a minimally invasive closure that leveraged the child’s robust PIEZO2 signaling to ensure proper vessel growth post‑surgery. Six months later, Javier was back on the soccer field, “feeling the wind on his face”—a reminder that knowledge truly saves lives.
Balancing risk and benefit in early interventions
When deciding whether to intervene surgically, doctors weigh the size of the defect, the child’s growth trajectory, and the genetic backdrop (including PIEZO2 status). A decision‑tree graphic—planned for the full article—will help families visualize options, from watchful waiting to catheter‑based repair.
Exercise Heart Metrics
Ever wondered why your doctor asks about “heart rate at the anaerobic threshold” during a treadmill test? It’s not just fancy jargon—those numbers are predictive powerhouses.
What does HR at the anaerobic threshold (HRAT) tell us?
A 2024 investigation of over 700 participants found that each single‑beat increase in HRAT cut the odds of having coronary artery disease (CAD) by 2.8 %. In plain English: a heart that can push a bit harder before hitting the “burn” zone is actually a healthier heart.
How is HR at the respiratory compensatory point (HRRCP) different?
HRRCP marks the moment the lungs start to over‑compensate for rising carbon dioxide. The same study showed a 2.6 % risk reduction per beat increase. Women, in particular, seemed to reap a bigger protective benefit, suggesting hormonal influences on autonomic control.
Dose‑response curve (visual cue)
Imagine a gentle hill—risk drops steadily as HRAT climbs, but beyond a certain point the slope flattens, indicating diminishing returns. This curve helps clinicians set realistic exercise targets for patients of all ages.
Practical take‑away for you
If you have access to a CPET (cardiopulmonary exercise test), ask your clinician to explain your HRAT and HRRCP results. Simple home‑based tools like a smartwatch can approximate these thresholds with the right software, empowering you to track progress.
Expert comment
Dr. Lena Morales, a cardiopulmonary physiologist, says, “HRAT is like a window into your autonomic balance. When it’s higher, your vagal tone is stronger, which we know protects against arrhythmias and atherosclerosis.”
Emerging Therapeutic Frontiers
We’re standing at the edge of a new era where the line between “basic science” and “clinical cure” is getting blurrier every day.
Mitochondrial modulation through exercise
A systematic review in Frontiers in Physiology (2023) highlighted that regular aerobic activity restores mitochondrial function, reducing oxidative stress—a key driver of heart failure. Think of mitochondria as tiny power plants; exercise is the maintenance crew that keeps them humming.
ADAR1‑mediated RNA editing
RNA editing, especially the A‑to‑I conversion performed by ADAR1, reshapes how genes are expressed in the heart. A 2023 review found that dysregulated ADAR1 activity contributes to inflammation and fibrosis. Small‑molecule ADAR1 modulators are now in early‑phase trials, offering a brand‑new class of heart‑protective drugs.
Setd7‑E2F1‑WWP2‑GPx4 pathway
In a 2025 Nature article, researchers discovered that the epigenetic enzyme Setd7 drives cardiac hypertrophy by degrading the antioxidant GPx4, leading to harmful lipid peroxidation. Targeting Setd7 with selective inhibitors halted hypertrophy in mouse models, opening a promising therapeutic avenue for patients with pressure‑overload heart disease.
Cardiovascular tissue engineering (CTE)
A bibliometric analysis spanning three decades (1992‑2022) revealed exponential growth in CTE research, with scaffold‑derived cardiomyocytes now achieving functional contractility close to native tissue. Imagine a future where a failing heart can be patched with a lab‑grown patch that beats in sync with your own rhythm.
Benefits vs Risks
Every scientific leap brings both excitement and caution. Let’s weigh them together.
Potential upside
- Earlier, non‑invasive detection of plaque and functional deficits.
- Personalized therapeutic regimens that minimize side‑effects.
- Regenerative options that could replace damaged myocardium.
Possible downsides
- Off‑target effects of gene‑editing tools (think “the unintended ripples”).
- Data‑privacy concerns with AI‑driven imaging platforms.
- High cost of novel drugs, potentially widening health disparities.
Risk‑mitigation checklist for clinicians
- Obtain thorough informed consent, highlighting uncertainties.
- Enroll patients in registries that track long‑term safety.
- Collaborate with multidisciplinary teams (genetics, imaging, pharmacy).
Stay Informed
Knowledge is the most empowering medicine we have. Here are a few ways to keep your heart‑health radar on point:
Top‑tier journals & databases
Stay current with PubMed alerts for “cardiovascular research” and follow the American Heart Association’s monthly updates. They curate the most reliable studies and translate them into plain language.
Trusted online resources
Websites like the European Society of Cardiology, the National Heart, Lung, and Blood Institute, and our own blog series on PIEZO2 ion channel provide vetted information without the hype.
Continuing‑education (CME) opportunities
In 2025‑2026, look for webinars on “Mechanosensitive Channels in Cardiology” and “RNA Editing Therapies.” Many are free for health professionals and often include Q&A sessions where you can ask the experts directly.
Whether you’re a patient, a caregiver, or a fellow health‑enthusiast, the whirlwind of cardiovascular research can feel overwhelming. But remember: each breakthrough—whether it’s a tiny ion channel, a new imaging algorithm, or a novel gene‑editing tool—starts with curiosity, just like the one that brought us together in this article.
What part of today’s research excites you the most? Have you tried any new heart‑healthy habits after learning about HRAT or PIEZO2? I’d love to hear your thoughts. Feel free to reach out, share your story, or ask a question—because together, we’re building a healthier future, one heartbeat at a time.


















Leave a Reply
You must be logged in to post a comment.