Living with chronic rhinosinusitis and those stubborn nasal polyps can feel like carrying a constant, muffled pressure in your head. You’ve probably tried sprays, pills, maybe even surgery, only to watch the polyps creep back. That’s where Dupixent (dupilumab) enters the picture—a biologic that’s quickly become the go‑to option for many patients who need more than a standard nasal spray. In the next few minutes, I’ll break down how Dupixent works, what the numbers say, how you take it, the side‑effects you might notice, and whether it could be a fit for you. Think of this as a chat over coffee, not a medical textbook.
How it works
What is Dupixent’s mechanism?
Dupixent is a lab‑crafted antibody that blocks two key messengers—IL‑4 and IL‑13—at the heart of type‑2 inflammation. Those cytokines are the culprits that drive the swelling, mucus, and tissue growth behind nasal polyps. By silencing them, Dupixent calms the immune over‑reaction without acting like a steroid, which means it doesn’t suppress your whole immune system.
Why is it different from steroids?
Oral or injected steroids flood your body with anti‑inflammatory chemicals, but they also raise blood sugar, can thin your bones, and often bring a roller‑coaster of side‑effects. Dupixent, on the other hand, is a targeted biologic; it zeroes in on the IL‑4/IL‑13 pathway while leaving the rest of your immune defenses largely intact. According to the FDA, it’s the first biologic approved specifically for chronic rhinosinusitis with nasal polyps (CRSwNP) source.
Trial results
How fast does it clear congestion?
The biggest surprise for many patients is speed. In the SINUS‑24 and SINUS‑52 phase‑3 trials, participants reported a noticeable drop in nasal congestion as early as Day 3, with statistically significant improvement by Week 4. By Week 24 the average least‑squares‑mean (LSM) reduction in congestion score was ‑0.87 compared with ‑0.12 for placebo; by Week 52 the gap widened to ‑0.98 versus ‑0.14 (p < 0.0001). That's roughly a 50 % improvement over standard care.
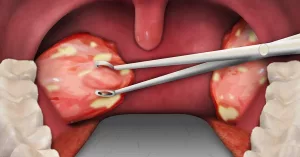
What about the polyps themselves?
Polyp size shrinks, too. The same trials showed a LSM change of ‑2.40 in the nasal polyp score (NPS) at Week 52—meaning a 37 % reduction versus a 5 % worsening in the placebo group. You can actually see the difference on endoscopic photos, with many patients describing “the polyps look like they’ve melted away.”
Does it lower surgery or steroid need?
Here’s the data that really matters to anyone who’s dreaded a repeat surgery: 83 % fewer Dupixent‑treated patients required sinus surgery, and 74 % needed fewer systemic steroids compared with placebo. Overall, there was a 76 % reduction in the combined need for surgery or steroids (hazard ratio 0.24) source.
| Endpoint | Dupixent (300 mg Q2W + INCS) | Placebo + INCS | % Improvement |
|---|---|---|---|
| Nasal Congestion (LSM) | ‑0.87 (wk 24) / ‑0.98 (wk 52) | ‑0.12 / ‑0.14 | ≈ 50 % |
| Nasal Polyp Score (LSM) | ‑2.40 (wk 52) | ‑0.15 | 37 % |
| Sinus Opacification (LMK‑CT) | ‑5.13 (wk 24) | ‑0.09 | 27 % |
| Surgery‑Free Survival (HR) | 0.17 | 1.00 | 83 % fewer |
| Systemic Steroid‑Free (HR) | 0.26 | 1.00 | 74 % fewer |
Dosage guide
What’s the prescribed schedule?
Dupixent comes as a pre‑filled syringe. The standard adult regimen is a 300 mg sub‑cutaneous injection every two weeks. Some doctors start with a loading dose of 600 mg (two injections on the same day) and then continue with the 300 mg Q2W schedule. The label also allows use in patients 12 years and older who meet the same disease criteria.
Can I combine it with other meds?
Yes—Dupixent is meant to be added on top of your usual intranasal corticosteroid (INCS) spray. Clinical data show that the combination yields the best results. There are no known drug‑drug interactions with common AR/Allergy meds, but always run a quick check with your provider.
Step‑by‑step injection checklist
- Wash your hands and clean the injection site with an alcohol swab.
- Pinch a small area of the upper thigh or abdomen (avoid the belly button).
- Insert the needle at a 90‑degree angle and press the plunger firmly.
- Hold for 5‑10 seconds, then remove the needle and apply light pressure.
- Dispose of the syringe in a sharps container.
Side effects
What are the most common reactions?
In the safety pool of over 700 patients, the most frequent side‑effects (≥ 1 % and higher than placebo) were:
| Side Effect | Dupixent % (n≈440) | Placebo % (n≈282) |
|---|---|---|
| Injection‑site reactions | 6 % | 4 % |
| Conjunctivitis | 2 % | 1 % |
| Arthralgia (joint pain) | 3 % | 2 % |
| Gastritis | 2 % | 1 % |
| Insomnia | 1 % | <1 % |
| Eosinophilia | 1 % | <1 % |
Any serious risks?
Serious but rare events have been reported, such as severe eye inflammation, eosinophilic pneumonia, or hypersensitivity reactions. In the trials, all cases of conjunctivitis resolved, and discontinuation due to adverse events was only 2 % versus 5 % in the placebo group. If you notice persistent eye redness, vision changes, or unusual swelling, call your doctor right away.
How does it compare to oral steroids?
While oral steroids can shrink polyps quickly, they bring a suite of long‑term issues—weight gain, mood swings, bone loss, and higher infection risk. Dupixent’s side‑effect profile is far milder and more focused, making it a safer long‑term maintenance option for many patients.
Who benefits
Which patients see the biggest gains?
Based on the trial populations and real‑world anecdotes (like Kathy’s story in the Dupixent videos), the sweet spot includes:
- Adults and teens (≥ 12 yr) with uncontrolled CRSwNP despite regular INCS.
- People who have already had sinus surgery but the polyps keep returning.
- Patients with comorbid asthma or NSAID‑exacerbated respiratory disease (NSAID‑ERD).
- Those who want to avoid repeated courses of systemic steroids.
When is it not recommended?
Dupixent isn’t for everyone. Avoid it if you are:
- Allergic to dupilumab or any of its ingredients.
- Pregnant or breastfeeding without discussing risks with your OB‑GYN (the data are still limited).
- Currently battling an active parasitic infection or about to receive a live vaccine.
Cost & assistance
What will it cost me?
In the United States, a 300 mg dose typically runs between $3,000 and $4,500 per month, depending on pharmacy pricing and insurance contracts. That sounds steep, but many patients qualify for the Dupixent MyWay® copay‑card, which can bring out‑of‑pocket costs down to under $100 per month for eligible individuals. You can request the card by calling the toll‑free support line or visiting the manufacturer’s assistance page here.
Will insurance cover it?
Most commercial insurers consider Dupixent a “step‑therapy” medication for CRSwNP, meaning they’ll require documentation of failed standard therapies (like INCS or a prior steroid course). A prior‑authorization package that includes recent CT scans, symptom scores, and a physician’s letter usually gets the green light. If you run into a denial, the manufacturer’s patient‑advocacy team can help you appeal.
Bottom line
If you’re exhausted by constant congestion, loss of smell, and the looming prospect of another sinus surgery, Dupixent offers a science‑backed, targeted alternative. The data show rapid relief—sometimes within days—steady shrinkage of polyps, and a dramatic cutback in steroid or surgical interventions. Side‑effects are generally mild, but as with any medication, a frank conversation with your ENT or allergist is essential.
Take a moment now: write down your current symptoms, note any past treatments, and bring that list to your next appointment. Ask your doctor if Dupixent is an option for you and whether you meet the criteria for financial assistance. You deserve breathing that feels natural again, and Dupixent may be the key to unlocking that relief.
Got questions or want to share your own Dupixent journey? Drop a comment below—let’s keep the conversation going and help each other breathe easier.
















Leave a Reply
You must be logged in to post a comment.