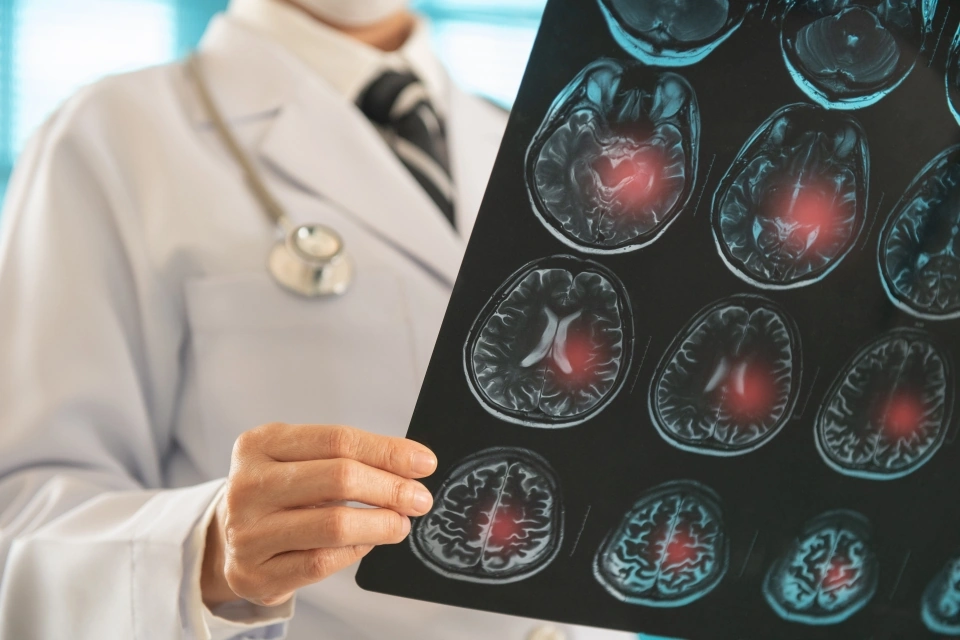
What Is Brain Ischemia
Imagine a tiny garden hose that suddenly gets kinked – the water can’t reach the thirsty plants, and they start wilting. That’s basically what happens in brain ischemia: a blood vessel narrows or blocks, cutting off oxygen and nutrients to brain cells. Within minutes, neurons begin to die, and the longer the blockage lasts, the bigger the “garden” that suffers.
Most often the culprit is a clot that forms in the heart or a large artery and travels to the brain. Less obvious causes include severe anemia, low blood pressure, or something as specific as sickle‑cell disease, which can cause tiny “sickled” cells to jam tiny vessels (sickle cell stroke risk).
Doctors gauge severity with the NIH Stroke Scale and imaging tests like CT or MRI. The mantra “time is brain” isn’t just a catchy slogan – studies show we lose roughly 1.9 million neurons each minute an occlusion remains untreated.
Acute Revascularization Basics
The first line of attack is revascularization: restore blood flow as quickly as possible. Two main tools are in the emergency toolbox.
IV thrombolysis (tPA) – a clot‑busting drug given through a vein. It works best within 4.5 hours of symptom onset. If you’re lucky enough to get it in time, chances of walking out of the hospital with minimal disability jump dramatically.
Mechanical thrombectomy – a tiny stent‑retriever snaked through the arteries to physically pull the clot out. Recent trials have stretched the window to 24 hours for selected patients, especially when advanced imaging shows a still‑viable “penumbra” around the core damage.
But there are trade‑offs. Thrombolysis can cause bleeding in the brain, and thrombectomy requires a specialized center and skilled interventionalists. Not everyone qualifies – about 80 % of acute ischemic stroke (AIS) patients end up with supportive care only (blood flow improvement strategies are critical for them).
According to a Frontiers in Neurology study (2020), even when arteries are reopened, some patients suffer “reperfusion injury” – a paradoxical damage caused by sudden oxygen rush. That’s why neuroprotective adjuncts are gaining attention.
Neuroprotective Strategies
Think of neuroprotection as giving the brain a safety blanket while the rescue crew works. It doesn’t replace revascularization, but it can soften the blow.
Normobaric Hyperoxia (NBO)
Simply put, breathe more oxygen. Delivering high‑flow oxygen (often 100 % via mask) raises the oxygen pressure in the penumbra without flooding the whole brain. A 2023 literature review in Heliyon found that short‑term NBO lowered in‑hospital mortality for AIS patients, and importantly, it didn’t boost harmful free‑radical production as once feared.
Therapeutic Hypothermia
Cooling the brain to 32‑35 °C slows metabolism, curbs excitotoxic glutamate release, and tampers down inflammation. Pre‑clinical work is compelling: mild hypothermia can shrink infarct size by over 40 % (Frontiers 2020). Human trials are trickier – keeping a conscious patient comfortably cool is a logistical puzzle. Still, the concept remains a beacon for future combination therapies.
Intermittent Hypoxia Conditioning (IH)
Sounds odd, but brief exposure to low‑oxygen breaths (think “mountain‑training for the brain”) triggers the body’s antioxidant defenses. A 2022 review in PMC highlighted that moderate IH ramps up enzymes like superoxide dismutase, giving neurons extra shield against the oxidative burst that follows reperfusion. The trick is dosing – too much hypoxia can be harmful, and individual factors (age, sex, existing comorbidities) shape the response.
Combination Approaches
Researchers are testing NBO plus hypothermia, or IH before/after thrombectomy, hoping the “team effort” will outpace the damage. Early animal data are promising, and a few pilot human studies are now enrolling – keep an eye on the clinical trial results page for updates.
Emerging Experimental Therapies
When conventional tools can’t reach every patient, scientists get creative.
Gene Therapy for Sickle‑Cell Stroke
CRISPR‑Cas9 and lentiviral vectors are being used to fix the faulty hemoglobin gene that causes sickle cells to harden and block vessels. The NIH‑funded UConn team, highlighted in a recent press release, received an R01 grant to push this forward. Early phase trials show reduced vaso‑occlusive events, and while the approach isn’t yet FDA‑approved, the gene therapy benefits could extend to lower stroke incidence in this high‑risk group.
Stem‑Cell & Extracellular‑Vesicle Therapy
Injecting mesenchymal stem cells or their tiny vesicle “mail‑packs” may deliver growth factors that nurture new blood vessels and dampen inflammation. Small pilot studies report modest improvements in motor scores, but larger randomized trials are still on the horizon.
Targeting the HIF Pathway
A 2024 article in Theranostics showed that activating the hypoxia‑inducible factor (HIF) rewires cellular metabolism, making neurons more tolerant to low‑oxygen stress. This metabolic switch could become a drug target, offering protection even before a clot forms.
Precision‑Stroke Care
Imagine an app that reads your genome, blood work, and imaging, then recommends the exact cocktail of clot‑buster, oxygen therapy, and maybe a gene‑editing schedule. AI‑driven models are already clustering patients into “responders” and “non‑responders,” paving the way for truly personalized brain ischemia treatment.
Balancing Benefits & Risks
Choosing the right path feels a bit like deciding whether to take a shortcut through a bustling city street or a longer, quieter alley. Both get you home, but one may have potholes you don’t see until it’s too late.
Decision matrix: time since onset, age, existing health conditions (like atrial fibrillation or sickle‑cell disease), imaging profile (size of core vs. penumbra), and patient preferences all weigh in. When the clock is ticking, the safest bet is rapid reperfusion; when the window has closed, neuroprotection and emerging therapies become the mainstay.
Common side‑effects to monitor:
- Intracranial hemorrhage from thrombolytics.
- Cooling‑related arrhythmias or shivering with hypothermia.
- Oxygen toxicity if NBO is prolonged beyond a few hours.
- Immune reactions in experimental gene or stem‑cell therapies.
If the risks outweigh the benefits, supportive care—blood pressure control, antiplatelet therapy, and early rehab—remains vital. Rehabilitation is where the brain’s plasticity shines, helping patients relearn skills even after significant damage.
Quick Takeaway
Brain ischemia treatment starts with a race against time: restore blood flow fast with IV tPA or a mechanical thrombectomy. While those are the cornerstones, adding neuroprotective layers—normobaric oxygen, mild cooling, or intermittent hypoxia—can protect the vulnerable penumbra. On the research frontier, gene therapy for sickle‑cell patients, stem‑cell releases, and HIF‑targeted drugs promise to broaden our arsenal. The key is balance—matching each patient’s unique profile with the right mix of proven and experimental options.
If you’re curious about how to boost circulation after a stroke, check out the article on blood flow improvement. For those wondering about the latest breakthroughs in genetics, the gene therapy benefits page is a good read. And of course, staying up‑to‑date with clinical trial results can give you a glimpse of what’s coming next.
We’ve covered a lot, but the most important message is this: even when a stroke feels like a sudden dark cloud, science is lighting up new paths every day. Stay informed, ask questions, and never underestimate the power of early, informed action. If you have thoughts or experiences you’d like to share, feel free to reach out – we’re all in this together.






















Leave a Reply
You must be logged in to post a comment.