Did you know the two FDA‑approved CAR‑T therapies for relapsed‑refractory multiple myeloma don’t work exactly the same way? Below you’ll get an at‑a‑glance comparison of Abecma and Carvykti, plus the hidden costs, side‑effect nuances, and the questions you should ask your doctor today. Let’s dive in—together.
Why Compare?
Choosing a treatment for multiple myeloma feels a bit like picking a new pair of shoes: you want something that fits perfectly, looks good, and won’t give you blisters later on. Both Abecma and Carvykti are powerful, but they each come with distinct benefits and trade‑offs. Understanding those differences helps you avoid “buyer’s remorse” and lets you have a confident, fact‑based conversation with your oncology team.
We’ll walk through the science, the numbers, and the real‑world experiences so you can see the whole picture—not just the glossy press release.
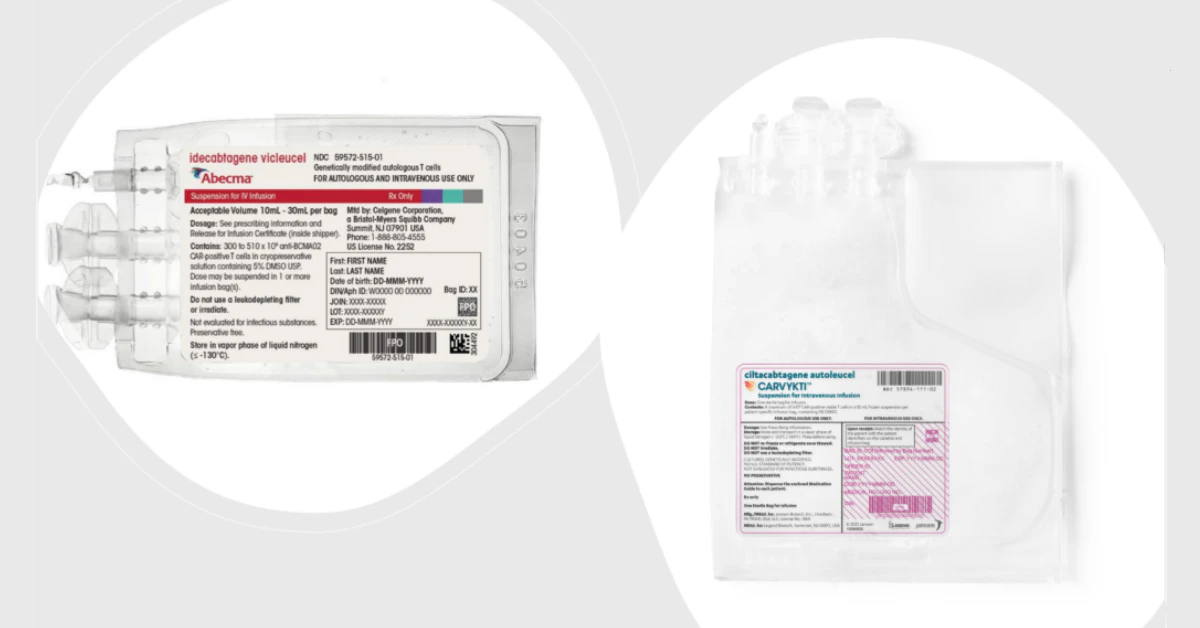
What Is Carvykti?
Mechanism of Action
Carvykti (ciltacabtagene autoleucel) is a CAR‑T therapy that teaches your own T‑cells to hunt down a protein called BCMA (B‑cell maturation antigen) that sits on myeloma cells. Once re‑engineered, the cells proliferate, locate the cancer, and unleash a targeted attack.
Regulatory Snapshot
The U.S. Food and Drug Administration gave Carvykti the green light in February 2022 for patients who have already tried at least four other lines of therapy—including an immunomodulatory agent, a proteasome inhibitor, and an anti‑CD38 monoclonal antibody.
Carvykti Price
According to the Carvykti price guide on Drugs.com, the list price hovers around $592,815 per unit. Insurance coverage, discount cards, and patient‑assistance programs can trim that number, but the headline figure is still eye‑watering. Keep this in mind when you talk finances with your care team.
What Is Abecma?
Mechanism of Action
Abecma (idecabtagene vicleucel) works on the same BCMA target, but its genetic construct is slightly different. Think of it as two different keys that open the same lock—one might turn smoother than the other depending on the lock’s wear and tear.
Regulatory Snapshot
The FDA approved Abecma earlier, in March 2021, for the same patient population (≥ 4 prior lines, including IMiD, PI, and anti‑CD38 therapy).
Abecma Price
Drugs.com lists the list price for Abecma at roughly $563,994 per unit. As with Carvykti, discount cards and insurance negotiations can lower out‑of‑pocket costs, but the baseline cost is still a major consideration.
Clinical Outcomes
When it comes to real‑world performance, the numbers speak louder than marketing copy. Below is a side‑by‑side snapshot of the most talked‑about metrics.
| Metric | Abecma (Ide‑cel) | Carvykti (Cilta‑cel) | Source |
|---|---|---|---|
| Overall Response Rate (ORR) | 60 % | 86 % | Real‑world analysis (ONC‑Live) |
| Median Progression‑Free Survival (PFS) | 7.5 months | Not reached (NR) | Same study |
| Severe CRS ≥ 3 | 2 % | 5 % (≈ 2.5× higher) | Health‑Tree retrospective table |
| Severe ICANS (Neurotoxicity) | 4 % | 0.6 % (delayed but 20× higher risk) | Health‑Tree retrospective table |
| Infection Rate | 35 % | 47 % (≈ 1.3× higher) | Health‑Tree retrospective table |
| Secondary Malignancies | 5 % | 9 % (≈ 1.8× higher) | Health‑Tree retrospective table |
In plain language: Carvykti often achieves deeper responses, but it also brings a higher chance of severe cytokine release syndrome (CRS) and delayed neuro‑toxicity. Abecma’s safety profile looks a touch gentler, which can matter a lot for older or frailer patients.
Safety Profile
Cytokine Release Syndrome (CRS)
CRS is like a feverish, over‑excited immune response that can cause high fever, low blood pressure, or even organ dysfunction. While both drugs can trigger CRS, Carvykti’s severe‑CRS rate (5 %) is more than double Abecma’s (2 %). Treatment typically involves tocilizumab (an IL‑6 blocker) and, if needed, steroids.
Immune Effector Cell‑Associated Neurotoxicity (ICANS)
ICANS can feel like confusion, tremors, or even seizures. The data show that Carvykti’s delayed neuro‑toxicity risk is roughly 20 times higher than Abecma’s, though the absolute numbers stay low (0.6 % vs. 4 %). Monitoring in an inpatient setting for at least 30 days after infusion is standard practice for both therapies.
Infections & Secondary Malignancies
Both CAR‑T therapies suppress the immune system, paving the way for bacterial, viral, or fungal infections. Carvykti’s infection rate of 47 % versus Abecma’s 35 % suggests you’ll need vigilant prophylaxis (antibiotics, antivirals, antifungals) and close follow‑up.
Secondary malignancies—including myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML)—are rare but real. Carvykti’s 9 % versus Abecma’s 5 % underscores the importance of long‑term surveillance.
Practical Considerations
Eligibility Checklist
- At least 4 prior therapy lines (IMiD, PI, anti‑CD38)
- ECOG performance status ≤ 2
- Adequate organ function (heart, liver, kidneys)
- No active uncontrolled infections
- Ability to wait 4‑6 weeks for manufacturing
Logistics & Administration
Both treatments require a “vein‑to‑vein” time of roughly 4–6 weeks while your T‑cells are harvested, engineered, and grown. The infusion itself occurs in a specialized center, followed by a minimum 30‑day inpatient monitoring period. Plan for a caregiver to stay nearby—hospital visits can be taxing.
Financial Navigation
Insurance pre‑authorization is a marathon, not a sprint. Bring the side‑effect table and cost comparison to your financial counselor. Many manufacturers offer “discount cards” that shave off a few thousand dollars; the Carvykti discount card and the Abecma discount card are a good place to start.
Quality‑of‑Life Snapshot
Patient‑reported outcomes from the pivotal KarMMa‑3 (Abecma) and CARTITUDE‑1 (Carvykti) trials show comparable improvements in fatigue and pain after response, but the higher toxicity rates with Carvykti can temporarily dip quality‑of‑life scores during the acute phase. In the long run, many patients report feeling “like themselves again” once remission is achieved.
Real‑World Stories
Case 1: The Energized 58‑Year‑Old
Mark, 58, was fit, active, and eager to get back to his weekend bike rides. After exhausting four prior regimens, his doctor recommended Carvykti. He achieved a complete response (CR) within two months. He did experience Grade 3 CRS, managed with tocilizumab, but recovered quickly and was back on his bike in three months.
Case 2: The Frail 72‑Year‑Old
Linda, 72, had heart‑failure concerns and a history of mild dementia. Her team leaned toward Abecma because of its slightly milder CRS profile. She achieved a very good partial response (VGPR) and, importantly, avoided severe neuro‑toxicity. She still needs regular visits, but her quality of life has markedly improved.
These anecdotes illustrate why “one size fits all” rarely applies in oncology. Your age, organ health, support system, and personal goals all shape the best choice.
Talk With Doctor
Armed with facts, you can steer the conversation toward meaningful answers:
- “Based on my health profile, which therapy do you think aligns best with my goals?”
- “What’s the realistic out‑of‑pocket cost after insurance and discount cards?”
- “How will my caregivers be involved during the inpatient stay?”
- “Are there clinical trials for next‑generation CAR‑T that might suit me?”
Pull the quick‑reference table you just read and hand it over. Seeing the numbers laid out can turn abstract risk percentages into a concrete discussion.
Future Directions
CAR‑T isn’t standing still. Bispecific antibodies (e.g., teclistamab) are already FDA‑approved, and next‑generation CAR‑T cells targeting two antigens simultaneously are entering early‑phase trials. A recent JCO retrospective analysis (JCO study) suggests ongoing refinement will keep pushing response rates higher while taming toxicities.
What does that mean for you? It means you have options today, and new options on the horizon. Staying informed and maintaining an open line with your oncologist ensures you can pivot when exciting data emerge.
Closing Thoughts
In the end, Abecma vs Carvykti is less about which drug “wins” and more about which one fits your unique story. Carvykti often delivers a higher response but carries a steeper risk of severe CRS and neuro‑toxicity; Abecma offers a gentler safety profile at a slightly lower price point.
Take this guide, discuss it with your care team, bring a trusted friend or family member to the appointment, and remember you’re not alone. The right decision is the one that balances effectiveness, safety, cost, and—most importantly—your personal values and life goals.
What’s your experience with CAR‑T therapy? Have you faced tough choices about cost or side effects? Share your thoughts in the comments—your story could help someone else navigating the same road.























Leave a Reply
You must be logged in to post a comment.