Why People Search
If you’ve ever stared at a glucose meter and felt a wave of frustration, you’re not alone. Thousands of people type “automated insulin delivery” into Google every day hoping to find a shortcut that makes blood‑sugar management feel less like a full‑time job. In a nutshell, the promise is simple: a device that watches your numbers, decides what your body needs, and delivers insulin without you having to lift a finger. The result? More time in the target range, fewer scary lows, and a little breathing room in an otherwise relentless routine.
Immediate Benefits
When an AID system does its job, you’ll notice three tangible improvements:
- Higher Time‑In‑Range (TIR) – most studies report an increase of 10‑15 % (roughly three extra hours of “good” glucose each day).
- Lower A1C – many users see a drop of 0.5 % or more within the first three months.
- Fewer extreme highs and lows – the algorithm can suspend insulin before a low and boost it before a high, cutting emergency visits.
Common Concerns & Risks
It’s not all sunshine and rainbows, though. Devices can malfunction, infusion sites can become irritated, and some people worry they’ll lose “feel” for their own diabetes. A recent substack article by Dan Heller warns that over‑reliance on automation may erode the critical self‑management skills many clinicians still consider essential.
Quick‑look Benefit vs Risk Table
| Benefit | Typical Impact | Potential Risk | Mitigation |
|---|---|---|---|
| Improved TIR | +10‑15 % | False‑readings from CGM | Calibrate sensor regularly |
| Lower A1C | ‑0.5 %+ | Device failure | Keep backup pump & glucose meter |
| Reduced hypo‑events | ‑40 % severe lows | Skin irritation | Rotate infusion sites every 2‑3 days |
How It Works
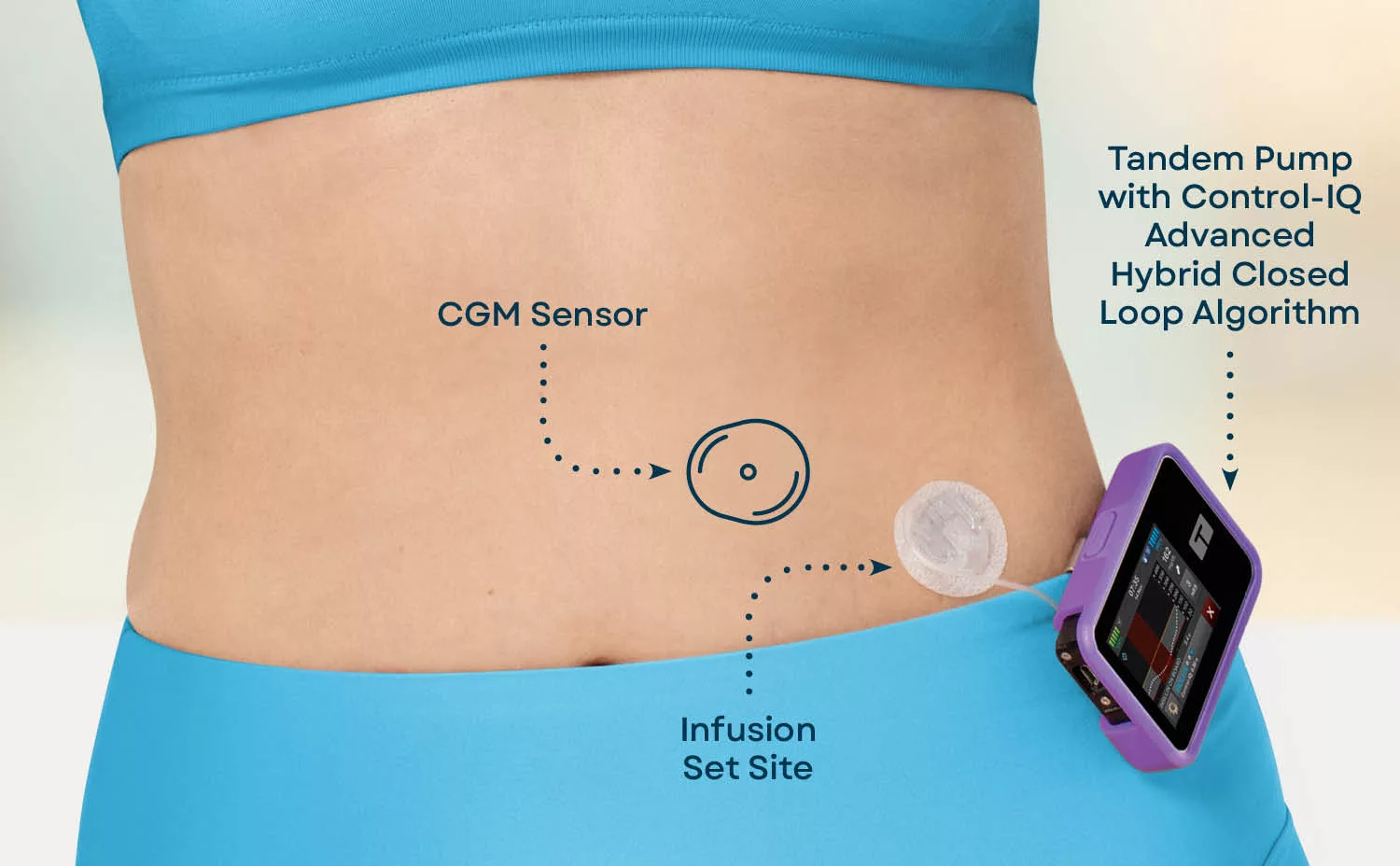
Think of an AID system as a tiny, tireless assistant that lives on your hip or waist. It has three core parts that talk to each other nonstop:
Core Components (the “triad”)
- Continuous Glucose Monitor (CGM) – a sensor that measures glucose in the interstitial fluid every five minutes and sends the data wirelessly.
- Insulin Pump or Pod – stores rapid‑acting insulin and can deliver basal doses continuously and bolus doses on demand.
- Control Algorithm – the brain that crunches the CGM data, predicts where glucose is headed, and tells the pump how much insulin to give.
Hybrid Closed‑Loop vs. Fully Automated
Most commercial systems are “hybrid” – you still announce meals and give a mealtime bolus, but the algorithm fine‑tunes basal delivery around that. A few DIY communities, such as OpenAPS, have built fully automated loops that can calculate corrections without any user input, but they require a fair amount of technical know‑how.
Algorithm Types
| Algorithm | Principle | Example System |
|---|---|---|
| Proportional‑Integral‑Derivative (PID) | Simple feedback based on current error | Medtronic MiniMed 780G |
| Model‑Predictive Control (MPC) | Projects glucose 30‑60 min ahead | Tandem Control‑IQ+ |
| Fuzzy‑Logic / Knowledge‑Based | Rule‑based “if‑then” decisions | Cambridge OpenAPS Loop |
Data Flow in Plain English
Imagine you’re driving a car with an autopilot. The CGM is the GPS, constantly updating your location. The algorithm is the driver‑assist software, deciding whether to press the gas (more insulin) or the brakes (less insulin). The pump is the engine, actually moving the car forward. Every five minutes the loop checks the road ahead and makes a tiny micro‑adjustment – keeping you cruising smoothly rather than constantly slamming the brakes.
Clinical Evidence
Words are nice, but numbers convince a lot of people. In the past five years, dozens of randomized controlled trials have tested AID systems. The 2023 European Association for the Study of Diabetes (EASD) consensus report pooled data from 12 RCTs and found that AID users achieved an average TIR of 74 % compared with 58 % for standard pump therapy. That’s the difference between spending a full three‑quarters of the day in the safe zone versus just over half.
What the Numbers Mean for You
Let’s translate those stats into everyday life. If you currently hit your target glucose 60 % of the time, an AID system could add roughly 60 extra minutes each day – that’s a whole lunch break without worrying about a sudden dip. For many, the most noticeable change is fewer night‑time lows. A study in Diabetes Spectr reported a 40 % reduction in severe hypoglycemia among adult participants using hybrid closed‑loop therapy.
Real‑World Story
Take Alex, a 28‑year‑old software engineer who switched to a Tandem Control‑IQ+ after years of finger‑stick chaos. Within three months his A1C fell from 8.2 % to 7.1 % and his nighttime lows dropped from eight episodes a month to just one. He says the biggest surprise was the “peace of mind” – he can now focus on coding rather than constantly checking alerts.
Choosing Your System
Every person with diabetes is unique, so the “best” AID system is the one that fits your lifestyle, budget, and medical needs. Below is a quick checklist you can run through during your next appointment.
Factors to Evaluate
- Age and Activity Level – Kids often need simpler parental controls; athletes may prefer rapid‑adjustment algorithms.
- CGM Compatibility – Some pumps only pair with Dexcom, others work with Freestyle Libre.
- Insurance Coverage – Check your plan’s formulary; out‑of‑pocket costs can vary dramatically.
- Desired Level of Automation – Hybrid, fully DIY, or hands‑off?
Comparison of Leading Systems
| System | FDA‑cleared? | CGM Required | Automation Level | Approx. Price (USD) |
|---|---|---|---|---|
| Tandem Control‑IQ+ | Yes | Dexcom G6/G7 | Hybrid | ≈ $7,000 |
| Medtronic MiniMed 780G | Yes | Guardian 3 | Hybrid | ≈ $8,000 |
| OpenAPS (DIY) | Open‑source | Any compatible CGM | Fully Automated | $0‑$1,000 (parts) |
When Semaglutide Enters the Conversation
If you’re managing type 2 diabetes with intensive insulin, adding semaglutide for blood‑sugar control can shrink the amount of basal insulin you need. That means the AID algorithm has a smaller “baseline” to work around, often resulting in smoother glucose curves. For people with type 1 diabetes, early research (semaglutide type 1 diabetes) suggests modest weight‑loss benefits and a slight reduction in total daily insulin dose, but always discuss this off‑label use with your endocrinologist.
Practical Tips
Even the smartest algorithm can’t save you from simple user errors. Here are some everyday habits that keep your system humming.
Set‑up & Calibration
- Insert the CGM sensor on a clean, dry site and follow the manufacturer’s calibration schedule (usually every 12 hours).
- Rotate infusion sites every 2‑3 days to avoid lipodystrophy – a condition where repeated insulin exposure damages the tissue, a point highlighted in Dan Heller’s risk analysis.
- Keep a spare set of batteries and an older‑generation glucose meter as a backup.
When Alerts Trigger
Alerts are your system’s way of saying, “Hey, I need a second opinion.” If you get a low‑glucose suspend warning, double‑check the sensor’s location and consider a temporary carbohydrate snack. For a high‑glucose correction, you can either let the algorithm deliver a correction bolus automatically (if you enabled that setting) or manually input a bolus using the pump’s interface.
Troubleshooting Quick‑Guide
| Symptom | Likely Cause | Fix |
|---|---|---|
| Pump stops delivering | Infusion set occlusion | Replace the set, check for kinks |
| CGM reads “error” | Sensor drift or poor contact | Remove, clean site, insert new sensor |
| Unexpected high‑glucose alerts | Carb counting mismatch | Re‑enter carbs, adjust insulin‑to‑carb ratio |
Balancing Automation with Self‑Knowledge
It’s tempting to hand over complete control, but staying engaged helps you spot trends that the algorithm might miss. Try reviewing your daily data every week: look for patterns like recurring highs after a certain workout or lows after a specific snack. Even a quick glance strengthens the partnership between you and your device.
Future Directions
The world of AID is evolving faster than most medical technologies. Researchers are feeding artificial‑intelligence models with millions of glucose‑insulin datapoints to create “personalized” algorithms that adapt to your unique physiology in real time. One exciting project from a data‑science lab (InsuLearner) uses machine learning to propose pump‑setting tweaks; early users report smoother curves with fewer manual adjustments.
Combining AID with GLP‑1 Agonists
Clinical trials listed on diabetes clinical trial registries are testing the combo of GLP‑1 drugs like semaglutide with AID. The logic is simple: semaglutide reduces post‑meal glucose spikes, leaving the AID algorithm to fine‑tune basal delivery rather than chase big swings. If these studies bear out, the future could hold a “dual‑action” regimen that lowers both A1C and body weight.
Regulatory Outlook (2025‑2027)
The FDA is moving toward “software as a medical device” pathways, meaning updates to algorithms could be delivered over‑the‑air without a new hardware recall. That could accelerate improvements and make it easier for smaller companies to bring niche solutions to market, especially for underserved populations.
Conclusion
Automated insulin delivery is far more than a flashy gadget—it’s a genuine lifestyle upgrade for many people living with diabetes. By boosting time‑in‑range, lowering A1C, and taking the edge off daily decision‑making, AID systems give you back precious minutes (and peace of mind) that you can spend on work, hobbies, or simply enjoying a cup of coffee without constantly checking your phone.
Choosing the right system, staying informed about risks, and keeping a hands‑on relationship with your data are the three pillars of success. If you’re curious about how AID fits into a broader type 1 diabetes treatment plan, or want to explore the potential of semaglutide for better glucose control, the resources are just a click away.
We hope this guide demystifies the technology and sparks confidence in making the next step. Have questions or a story of your own? Feel free to reach out—sharing experiences is how we all learn and grow together.


















Leave a Reply
You must be logged in to post a comment.