Imagine being able to sprinkle a few tiny, engineered “mini‑pancreases” into your body and watch them quietly keep your blood sugar in check, day after day. That’s the promise of 3D bioprinting islets—a technology that’s nudging us closer to a long‑term cure for type 1 diabetes. In this article we’ll walk through what the science looks like today, why it matters for you, and what hurdles still need clearing. Grab a coffee, settle in, and let’s explore this exciting frontier together.
Quick Answer
In a nutshell, 3D bioprinting islets are lab‑crafted clusters of insulin‑producing cells printed layer by layer inside a supportive “bioink.” Early animal studies show they can normalize blood glucose for weeks without daily injections, and clinical trials are on the horizon. Think of it as a custom‑made, transplant‑ready pancreas—only smaller, safer, and potentially limitless.
What Are Islets
Definition and Terminology
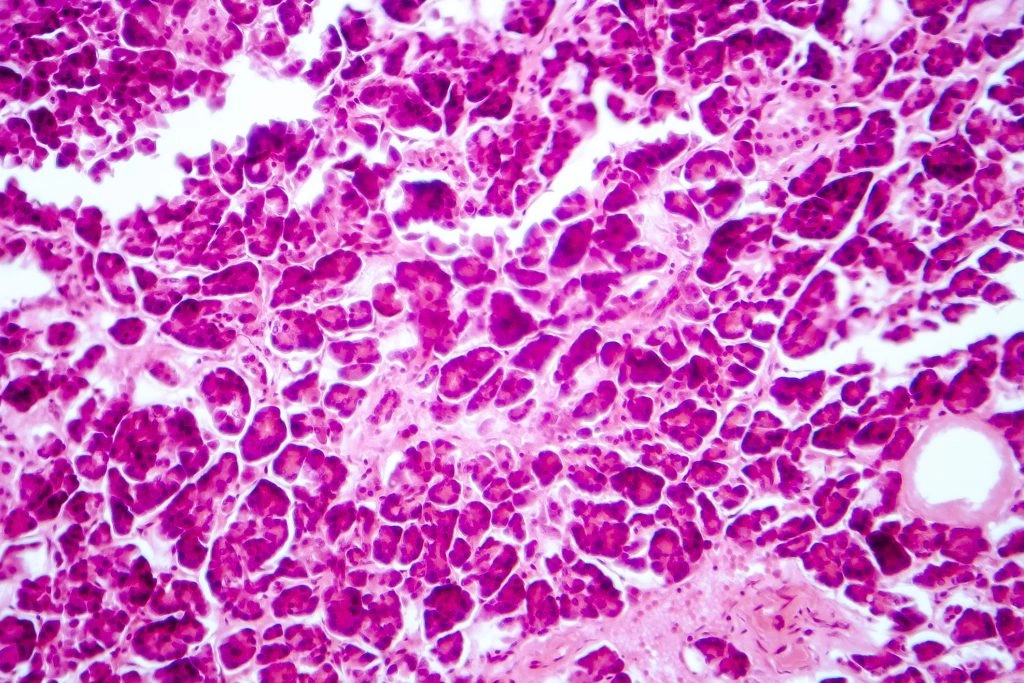
When we talk about “islets,” we mean the tiny groups of beta‑cells tucked throughout the pancreas that release insulin in response to rising blood sugar. 3D bioprinting islets (sometimes called 3D printed islets) are not harvested from donors; they’re built from stem cells or donor cells using a printer that deposits cells and biomaterials with micrometer precision.
How They Differ From Natural Islets
Natural islets sit inside a rich extracellular matrix (ECM) that supplies nutrients, signals, and a vascular network. A 3D‑printed construct tries to mimic that niche by embedding the cells in a specially formulated bioink for diabetes and arranging tiny channels that invite blood vessels to grow.
| Feature | Native Islet | 3D‑Bioprinted Islet |
|---|---|---|
| Cell Source | Donor pancreas | Stem‑cell-derived or donor‑derived cells |
| Support Matrix | Native ECM | Engineered ECM‑rich bioink (e.g., PINE) |
| Vascular Integration | Pre‑existing vessels | Printed micro‑channels + angiogenic cues |
| Scalability | Limited by donors | Potentially unlimited, on‑demand |
Science Behind Print
What Is “Bioink for Diabetes”?
Think of bioink as the “dough” a 3D printer uses. For diabetes, researchers blend gelatin, collagen, laminin, and other ECM proteins into a paste that keeps cells alive and nudges them toward a functional beta‑cell phenotype. The POSTECH team even named their formulation PINE (Peri‑Islet Niche‑like ECM) because it recreates the exact surroundings beta‑cells love.
Cell Sources: Stem vs. Donor
Stem‑cell‑derived beta‑like cells offer an inexhaustible supply, but they need a nurturing environment to mature. Donor cells, on the other hand, are already primed but scarce. Many labs are testing hybrid approaches—seed donor islets inside a printed scaffold and bolster them with supportive stromal cells, as shown in a recent study that used adipose‑derived stromal cells to preserve function.
Printing the Vascular Niche
Insulin‑secreting cells are hungry for oxygen. To solve that, researchers print microscopic channels and sprinkle angiogenic growth factors (VEGF, FGF) into the bioink. In animal models, these channels quickly become perfused with host blood vessels, delivering nutrients just like a real pancreas.
Latest Breakthroughs
Bioprinted Pancreatic Petals
A 2023 pre‑clinical study placed 3D‑bioprinted “petals” containing porcine islets under mouse skin. The grafts lowered fasting glucose from ~140 mg/dL to the low 110s within two weeks and stayed stable for a month—a clear win over traditional renal‑capsule transplants.
POSTECH’s HICA‑V Platform (2025)
According to a Nature Communications paper, a team at Pohang University printed a Human Islet‑like Cellular Aggregate and Vasculature (HICA‑V). Their printed islets not only produced more insulin than controls but also reacted to inflammatory cues—an essential step toward personalized disease modeling.
Comprehensive Review (2023)
The Exploration of Medicine review summed up over 200 papers, highlighting how bioink composition, cell source, and printing resolution all influence graft success. The authors pointed out that integrating immune‑shielding layers could finally let us skip lifelong immunosuppression.
Treatment Impact
Why Current Therapies Fall Short
Insulin pumps and daily injections are lifesaving, yet they can’t fully mimic the rapid spikes and dips native beta‑cells manage. Whole‑organ transplantation solves the problem but is limited by donor scarcity and the need for heavy immunosuppression. Type 1 diabetes treatment options remain fragmented, and many patients still face daily uncertainty.
Potential Advantages of a Bio‑Artificial Pancreas
- Long‑Term Regulation: Printed islets can sense glucose continuously and release insulin on demand.
- Personalized Graft Size: The printer tailors the dose to each patient’s body weight and metabolic rate.
- Reduced Immunosuppression: Encapsulation or immune‑protective bioinks could shield the graft.
- Scalable Production: No more waiting for donor organs.
Clinical‑Trial Pipeline
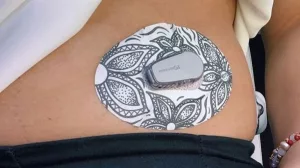
The first human safety trial is slated for early 2025, enrolling adults with brittle type 1 diabetes who have struggled with hypoglycemia unawareness. Researchers will implant a small plug‑and‑play device under the abdominal skin and monitor glucose for six months.
Benefits ↔ Risks
| Aspect | Benefit | Risk / Challenge |
|---|---|---|
| Graft Survival | Weeks‑to‑months of stable insulin output | Ensuring rapid vascularization |
| Immunogenicity | Potential for immune‑shielded constructs | Possibility of rejection without adequate protection |
| Scalability | On‑demand, patient‑specific production | Consistent bioink quality at scale |
| Safety | Biodegradable polymers reduce long‑term foreign body response | Long‑term biocompatibility still under study |
Balancing these factors is why many labs pair islets with stromal cells, as the 2023 adipose‑derived study showed a 30 % boost in insulin secretion and less oxidative stress.
Therapy Comparison
Encapsulation Devices
Devices like Theracyte trap islets inside a semi‑permeable membrane. They protect cells from immune attack but can limit nutrient flow, leading to slower insulin response.
Gene‑Edited Stem‑Cell Therapies
CRISPR‑edited stem cells aim to produce immune‑evading beta‑cells. Promising, yet the regulatory pathway is still winding.
Traditional Donor Islet Transplantation
Transplanting isolated islets under the kidney capsule works but requires multiple donors for one recipient and lifelong immunosuppression.
| Therapy | Efficacy (short‑term) | Cost | Scalability |
|---|---|---|---|
| Encapsulation | Modest glucose control | Medium | Medium |
| Gene‑Edited Stem Cells | High (experimental) | High | Potentially high |
| Donor Islet Tx | High (limited) | Very high | Low (donor shortage) |
| 3D‑Bioprinted Islets | Promising (animal data) | Currently high, expected lower | High (on‑demand) |
Future Outlook
Regulatory Roadmap
The FDA has granted “Breakthrough Device” status to a few bioprinting platforms, signaling a faster path to market if safety data hold up.
Upcoming Human Trials
Beyond the 2025 safety study, a multicenter Phase II trial will test efficacy in a broader population, measuring time‑in‑range (TIR) and quality‑of‑life scores.
How You Can Prepare
- Stay informed through reputable diabetes research newsletters.
- Consider enrolling in trial registries if you meet the criteria.
- Discuss emerging options with your endocrinologist—knowledge is power.
Conclusion
From the first ink‑droplet on a printer bed to a living, insulin‑secreting cluster, 3D bioprinting islets are rapidly moving from sci‑fi imagination to realistic therapy. While challenges around vascularization, immune protection, and large‑scale manufacturing remain, the momentum is undeniable. If you or a loved one lives with type 1 diabetes, these advances mean a future where daily injections could become a relic of the past. Keep your eyes on the research, ask your doctor about upcoming trials, and remember—science is powered by curious minds just like yours. Together, we’re shaping a world where diabetes is no longer a life‑long burden.



















Leave a Reply
You must be logged in to post a comment.