Imagine you could catch a problem before it even shows up on a scan, or know exactly which medicine will work for you without a trial‑and‑error dance. That’s the promise of colorectal cancer biomarkers – tiny biological clues that tell us about risk, early disease, and the best way to treat it. In the next few minutes we’ll walk through what these biomarkers are, why they’re becoming a game‑changer, and how you can talk about them with your doctor. Grab a cup of tea, settle in, and let’s explore this fascinating world together.
What Are Biomarkers
Definition in plain language
In the simplest terms, a biomarker is a measurable sign that lives inside your body – a piece of DNA, a protein, a cell, or even a gut microbe – that signals something is happening. When it comes to colorectal cancer (CRC), biomarkers can reveal whether a tumor is present, how aggressive it might be, or whether a particular therapy will hit its target.
Types we use for CRC
There’s a colorful menu of biomarkers that doctors can order:
- Genetic mutations – changes in genes such as KRAS, BRAF, or the mismatch‑repair genes that cause Lynch syndrome.
- Epigenetic markers – DNA methylation patterns like NDRG4 and BMP3 that show up in stool or blood.
- Protein & circulating markers – circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) that drift through your bloodstream.
- Stool‑based DNA – multi‑target stool DNA (mt‑sDNA) tests such as Cologuard that hunt for mutated DNA shed in feces.
How they’re measured
Traditionally, doctors examined tissue from a colonoscopy. Today, you can get a liquid biopsy – a simple blood draw – or a stool sample collected at home. Each method has trade‑offs. Blood tests are quick and minimally invasive, but they may miss tiny lesions. Stool DNA tests capture shed cells from the entire colon but can be a bit messier to process. Below is a quick comparison:
| Test Type | Sample | Invasiveness | Typical Sensitivity (Stage I‑II) | Typical Specificity |
|---|---|---|---|---|
| Colonoscopy (tissue biopsy) | Colon tissue | Invasive, sedation required | ≈ 95 % | ≈ 99 % |
| Liquid biopsy (ctDNA/CTC) | Blood | Minimally invasive | 70‑85 % | 80‑90 % |
| Stool DNA (mt‑sDNA) | Stool | Non‑invasive, at‑home | ≈ 92 % | ≈ 84 % |
| FIT (fecal immunochemical test) | Stool | Non‑invasive | ≈ 70 % | ≈ 95 % |
Why They Matter
Early detection power
Detecting CRC when it’s still curable can shave years off a patient’s life expectancy. According to circulating tumor DNA (ctDNA) studies, blood‑based biomarkers pick up stage I disease in about three‑quarters of cases – a huge leap over FIT alone. The Early Detection Research Network even frames these markers as “the next frontier for population screening” (Early Detection Research Network framework).
Personalizing treatment
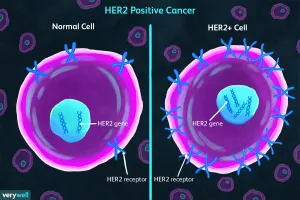
Not all colorectal cancers respond to the same drugs. If your tumor is KRAS wild‑type, anti‑EGFR antibodies like cetuximab can be lifesavers. On the other hand, tumors with high microsatellite instability (MSI‑H) often respond spectacularly to immunotherapy. This is what we call biomarker‑guided treatment – the right drug for the right molecular profile.
Assessing hereditary risk
About 35 % of CRC cases have a genetic component (genetic and stool‑based biomarkers). If you have a family history of Lynch syndrome or a known APC mutation, testing for these heritable cancer markers can guide earlier, more frequent screening, sometimes starting in your twenties.
Reducing unnecessary procedures
Imagine a scenario where a positive stool‑DNA test flags a high‑risk individual, prompting a colonoscopy that finds and removes a precancerous polyp. Conversely, a negative biomarker panel can reassure low‑risk patients and spare them an invasive exam. This “risk‑stratified” approach saves both money and anxiety.
Standard vs Emerging
Conventional screening tools
FIT, colonoscopy, and sigmoidoscopy have saved countless lives, but they’re not perfect. FIT can miss non‑bleeding lesions; colonoscopy, while thorough, is costly, requires bowel prep, and carries a small perforation risk.
FDA‑approved biomarker tests
Several tests have cleared regulatory hurdles:
- Cologuard – a multitarget stool DNA test that combines FIT with DNA markers.
- Epi proColon – a blood test that detects methylated DNA from cancer cells.
- Guardant360 – a comprehensive liquid biopsy panel covering dozens of mutations.
Research‑stage candidates
Scientists are peeking into the gut’s microbial world, spotting bacteria like Fusobacterium nucleatum as early warnings (recent review of CRC biomarkers). Another bold experiment is the polymer‑pattern blood test that claims 72 % sensitivity for colon polyps (polymer‑pattern analysis study). While promising, these still need large‑scale validation before they hit the clinic.
Speed to clinic
Typical biomarker development follows a pipeline: discovery → analytical validation → clinical validation → regulatory review. Most emerging tests are in the “clinical validation” phase, meaning they’re being tested in real‑world patients but haven’t yet secured FDA approval.
Reading Your Results
Positive vs. negative
A “positive” result means the test detected a marker above a set threshold. It doesn’t guarantee cancer, but it raises the suspicion enough that a colonoscopy is usually recommended. A “negative” result suggests low likelihood, yet it’s not an absolute zero – especially if you have a strong family history.
When results are “inconclusive”
Some labs report “borderline” levels. In those cases, repeat testing after a few weeks or moving straight to imaging can clear the picture. It’s perfectly okay to ask your doctor for the specific cut‑off values used – transparency builds trust.
Next steps after a positive
1. Schedule a colonoscopy (the gold‑standard confirmation).
2. Discuss potential genetic counseling if hereditary markers were found.
3. Review lifestyle factors – diet, exercise, smoking – that could modify risk.
4. Consider enrollment in a clinical trial if your tumor has a rare mutation.
Emotional impact
Finding a biomarker can feel like a punch to the gut. It’s normal to experience anxiety, anger, or even denial. Talking to a support group, a therapist, or a trusted friend can help you process the news. Remember, a biomarker is a tool, not a verdict.
Talk to Your Doctor
Preparing for the visit
Write down these questions before you walk into the exam room:
- Which biomarker tests are appropriate for my age and family history?
- What will the test cost, and will my insurance cover it?
- If the result is positive, what are the exact follow‑up steps?
- Are there any lifestyle changes that could improve my biomarker profile?
Understanding insurance
Most major insurers cover colonoscopy when a high‑risk biomarker test is positive. However, coverage for liquid biopsies can be spotty. Ask the billing department for CPT codes – they’re the secret language insurers understand.
When to seek a second opinion
If you’re offered an expensive test that isn’t widely endorsed, or if the interpretation feels vague, a second opinion from a gastro‑oncologist or a clinical genetics specialist can give you clarity. A good doctor welcomes those questions.
Shared decision‑making
Decisions about screening are personal. According to the American Society of Clinical Oncology, patients who actively participate in their care tend to have better outcomes and higher satisfaction. So, feel empowered to voice your preferences.
Future Outlook
Multi‑omics panels & AI
Researchers are training artificial‑intelligence models to read dozens of biomarkers at once – DNA mutations, methylation patterns, protein levels, and even gut‑microbiome signatures. Early simulations suggest these panels could cut CRC mortality by up to 30 % when used for population screening.
Home‑based liquid‑biopsy kits
Imagine ordering a small finger‑prick kit, sending a blood spot to a lab, and receiving a risk report in a week. Several startups are piloting this, but regulatory hurdles remain – accuracy must be rock‑solid before we see them on pharmacy shelves.
Population screening with combined stool‑DNA & microbiome
Combining traditional stool DNA with microbiome analysis (looking at bacteria like Fusobacterium) may increase sensitivity beyond 95 %. Modeling studies predict that such a hybrid test could reduce late‑stage diagnoses dramatically.
Clinical‑trial opportunities
If you test positive for a rare mutation, you might qualify for a targeted‑therapy trial. Websites like ClinicalTrials.gov list thousands of active studies – a quick search for “colorectal cancer biomarker” can reveal options near you.
Bottom‑Line Summary & Call‑to‑Action
Let’s recap the three big takeaways:
- Biomarkers catch cancer early. Whether it’s a blood draw, a stool sample, or a tissue test, these tiny signals give doctors a head start.
- They personalize treatment. Knowing your tumor’s genetic makeup means you can avoid ineffective drugs and get the right therapy faster.
- They empower you. With the right questions, you can shape a screening plan that fits your risk, your lifestyle, and your peace of mind.
If you’re curious about whether a biomarker test is right for you, start the conversation at your next check‑up. Download the printable “Biomarker Discussion Checklist” below, share it with your doctor, and take charge of your colorectal health.
Got questions or personal experiences with biomarker testing? I’d love to hear them in the comments. Together we can demystify these tests and make informed choices that keep us healthy for years to come.


















Leave a Reply
You must be logged in to post a comment.