Hey there! If you or someone you love has been diagnosed with neuromyelitis optica spectrum disorder (NMOSD), you’ve probably seen the name Enspryng pop up a lot. You’re likely wondering what it actually does, how you take it, how much it costs, and whether it’s safe. Below is a friendly, down‑to‑earth rundown that gives you the answers you need without the medical‑jargon overload. Let’s dive in together.
Quick Answer Snapshot
What is Enspryng? It’s a prescription biologic called satralizumab, approved by the Enspryng fda approval to treat adults with aquaporin‑4 (AQP4) antibody‑positive NMOSD. It works by blocking interleukin‑6 (IL‑6) receptors, which calms the immune system and reduces the chance of a relapse.
How do you take it? After a short loading phase, you give yourself a subcutaneous injection once a month—usually in the abdomen or thigh. No clinic visits needed after you’re trained.
Is it affordable? Prices vary, but many patients qualify for insurance help or a Genentech co‑pay program. Check out the Enspryng price page for the latest figures and assistance options.
How it Works
IL‑6 pathway and NMOSD
NMOSD is driven by a misguided immune attack on the water channel protein aquaporin‑4, which sits on the surface of nerve cells in the optic nerves and spinal cord. One of the key messengers that fuels this attack is IL‑6, a cytokine that tells immune cells to ramp up production of harmful antibodies.
Enspryng contains a monoclonal antibody that binds to the IL‑6 receptor, effectively putting a “mute” button on the signal. By silencing IL‑6, the drug lowers the production of AQP4‑targeting antibodies and helps protect the nervous system from further damage.
Clinical evidence
Two pivotal phase III trials—SAkuraStar and SAkuraSky—showed that patients on Enspryng experienced a 50‑70% reduction in relapse risk compared with placebo. The median time to first relapse stretched beyond a year, and safety data now extend beyond five years of follow‑up, confirming a solid benefit‑risk profile (FDA).
Simple flow‑chart (you can picture it)
Think of the immune system as a loudspeaker. IL‑6 is the volume knob. Enspryng turns that knob down, quieting the “noise” that leads to antibody attacks on AQP4.
Who Can Use
FDA‑approved indication
The drug is specifically approved for adults (≥ 18 years) who test positive for AQP4 antibodies. If you’re AQP4‑negative, your neurologist might consider alternative therapies.
Contra‑indications & precautions
- Known allergy to satralizumab or any excipient.
- Active hepatitis B infection.
- Untreated latent tuberculosis (TB).
- Live or live‑attenuated vaccines within four weeks of dosing.
Pre‑treatment labs & vaccinations
Before the first dose, doctors usually order liver‑function tests, a complete blood count (to check neutrophils), and hepatitis B/TB screens. Vaccinations—especially flu and COVID‑19—should be up to date, but give a non‑live vaccine at least two weeks before starting Enspryng.
Checklist for patients
| Item | What to Do |
|---|---|
| Blood work | Get liver enzymes, CBC, Hep B, TB test |
| Vaccines | Complete non‑live shots >2 weeks before first dose |
| Allergy check | Confirm no satralizumab hypersensitivity |
| Insurance paperwork | Submit prior‑authorization with dosing schedule |
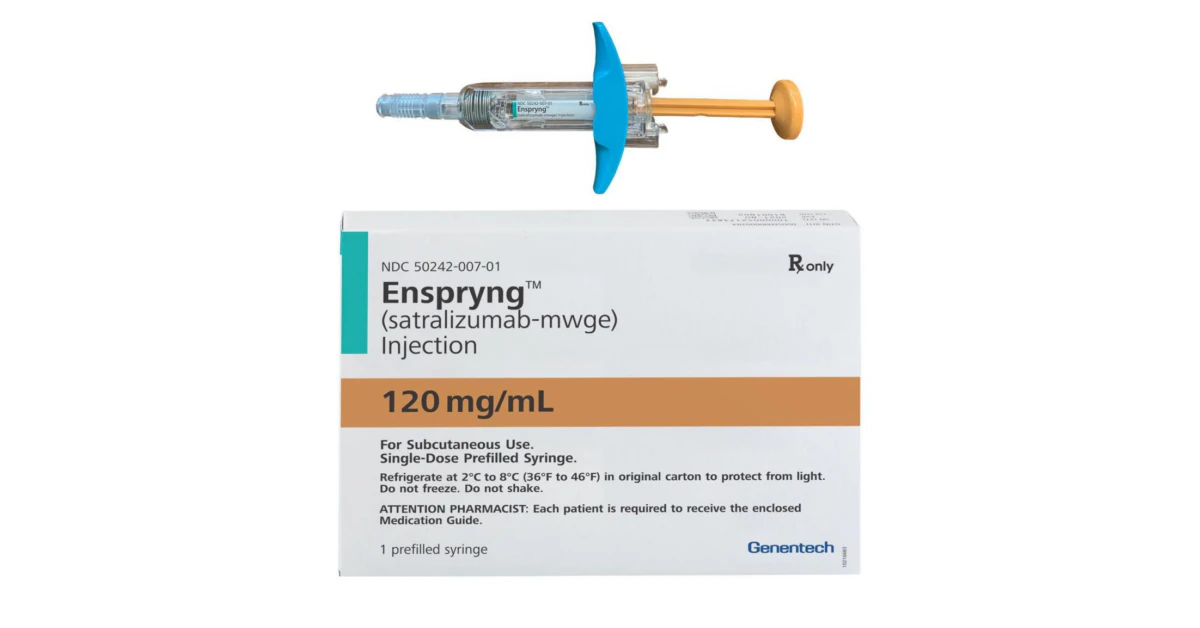
Dosing & Injection
Loading phase vs. maintenance
Enspryng comes in a prefilled 120 mg syringe. The schedule looks like this:
- Week 0: First injection
- Week 2: Second injection
- Week 4: Third injection (now you’ve completed the “loading” phase)
- Every 4 weeks thereafter: Maintenance dose
This pattern gives your body a steady level of the drug while it learns to keep the immune response in check.
Step‑by‑step injection
Here’s a quick, friend‑to‑friend guide for your monthly self‑shot:
- Wash your hands and find a clean surface.
- Pick an injection site—either the abdomen (avoid the belly button) or the outer thigh.
- Rotate sites each month to prevent skin irritation.
- Remove the cap, check that the solution is clear, and pinch the skin gently.
- Insert the needle at a 90‑degree angle, push the plunger fully, then withdraw.
- Dispose of the syringe in a sharps container.
If you need a visual refresher, our enspryng injection guide walks you through each step with screenshots.
Tips for staying on track
Life gets busy, but missing a dose can compromise your protection. Here are a few tricks that work for many patients:
- Mark the injection day on your calendar (or set a phone alarm).
- Keep a simple diary noting the date, site, and any reaction.
- Pair the reminder with a regular habit—like taking a weekly vitamin.
- Ask your pharmacist for a refill reminder card.
Price & Assistance
Typical list price
In the United States, the wholesale acquisition cost hovers around $12,000–$13,000 per 120 mg syringe. Canada and European markets have their own pricing structures, often a bit lower due to national health‑plan negotiations.
Insurance coverage & prior‑auth tips
Most commercial insurers cover Enspryng, but they usually require a prior‑authorization. When you or your clinic submits the request, include:
- Diagnosis code for NMOSD (ICD‑10 G36.0).
- Lab proof of AQP4‑positivity.
- Documentation of prior therapies (if any) and why Enspryng is the next step.
Having a concise “letter of medical necessity” ready can speed up approval.
Co‑pay and patient‑support programs
Genentech runs a co‑pay assistance program that can offset up to $20,000 per calendar year for eligible commercially insured patients. Enrollment is online, and you’ll need your prescription details handy.
Learn more about costs
For a deep dive into current pricing, insurance tricks, and how to apply for help, see our Enspryng price article.
Safety Profile Overview
Common side effects
In clinical studies, more than 5 % of patients reported at least one of the following:
- Nasopharyngitis (runny nose, sore throat)
- Rash or mild skin irritation
- Fatigue or general tiredness
- Headache
- Joint pain (arthralgia)
- Gastritis or mild stomach upset
Most of these are mild and resolve on their own or with simple measures (e.g., acetaminophen for headache).
Serious risks to monitor
Because Enspryng dampens part of the immune system, keep an eye out for:
- Serious infections (e.g., pneumonia, cellulitis). Call your doctor right away if you develop fever, chills, or an unexplained cough.
- Hepatitis B reactivation—regular blood tests are essential.
- Elevated liver enzymes—your provider will schedule periodic labs.
- Neutropenia (low white‑blood‑cell count), which can increase infection risk.
- Rare, severe allergic reactions (anaphylaxis). Seek emergency care if you notice swelling of the face, difficulty breathing, or hives after an injection.
Real‑world patient story
Take Jennifer, a 42‑year‑old teacher who started Enspryng in early 2023. She says the first two injections gave her a mild injection‑site itch, but after the loading phase she’s been relapse‑free for 14 months. “I love that I can give the shot at home while watching my kids’ homework,” she laughs. Her experience illustrates the balance: a few manageable side effects, big payoff in quality of life.
When to call your doctor
- Fever > 100.4 °F (38 °C) lasting > 24 hours.
- Persistent cough, shortness of breath, or chest pain.
- Yellowing of skin or eyes (possible liver issue).
- Rapid swelling or severe rash at the injection site.
- Any new neurological symptoms (e.g., vision changes, weakness).
Talk to Your Doctor
Preparation makes the conversation smoother. Bring these items to your next appointment:
- Recent MRI or imaging reports.
- Lab results showing AQP4 positivity and baseline liver/renal function.
- List of current medications (including over‑the‑counter supplements).
- Questions about vaccination timing, insurance coverage, and co‑pay assistance.
- Printed copy of the enspryng mechanism of action summary if you want to discuss how it fits with other treatments.
Don’t hesitate to ask how the loading schedule fits into your lifestyle, or whether you need extra monitoring because of a past infection. A transparent, two‑way dialogue builds trust and ensures you feel empowered in your treatment journey.
Bottom‑Line Takeaways
Enspryng (satralizumab) offers a convenient, once‑monthly home injection that can dramatically lower relapse risk for adults with AQP4‑positive NMOSD. Its mechanism—blocking IL‑6 receptors—addresses the disease at a core immune‑signaling level, and robust trial data back up its efficacy. While the drug is not cheap, many patients benefit from insurance coverage, co‑pay assistance, and patient‑support programs. Side‑effects are generally mild, but serious infections and liver concerns require regular monitoring.
Ultimately, the decision to start Enspryng should be made with your neurologist, weighing the benefits of reduced disease activity against the vigilance needed for safety. If you’re curious about cost, have questions about injection technique, or just need reassurance, explore the internal resources we linked throughout this article. You deserve a treatment plan that feels both effective and manageable.
We hope this guide has cleared up the fog around Enspryng. If you’ve had personal experience with the medication—or if you have lingering questions—feel free to share your thoughts. Knowledge is power, especially when navigating a rare condition like NMOSD. Stay hopeful, stay informed, and remember: you’re not alone on this path.


















Leave a Reply
You must be logged in to post a comment.