Enspryng (satralizumab) is a humanized monoclonal antibody that blocks the interleukin‑6 (IL‑6) receptor, slowing the inflammatory fire‑storm that fuels neuromyelitis optica spectrum disorder (NMOSD). In plain language: it stops a key “conversation” between immune cells that would otherwise tell the body to attack the nervous system. By silencing that conversation, relapses become far less frequent and patients can reclaim a steadier, brighter tomorrow.
Want the basics on pricing or how to inject it? Check out our Enspryng overview, the prescribing information, and the latest FDA approval details. All of those pages live just a click away.
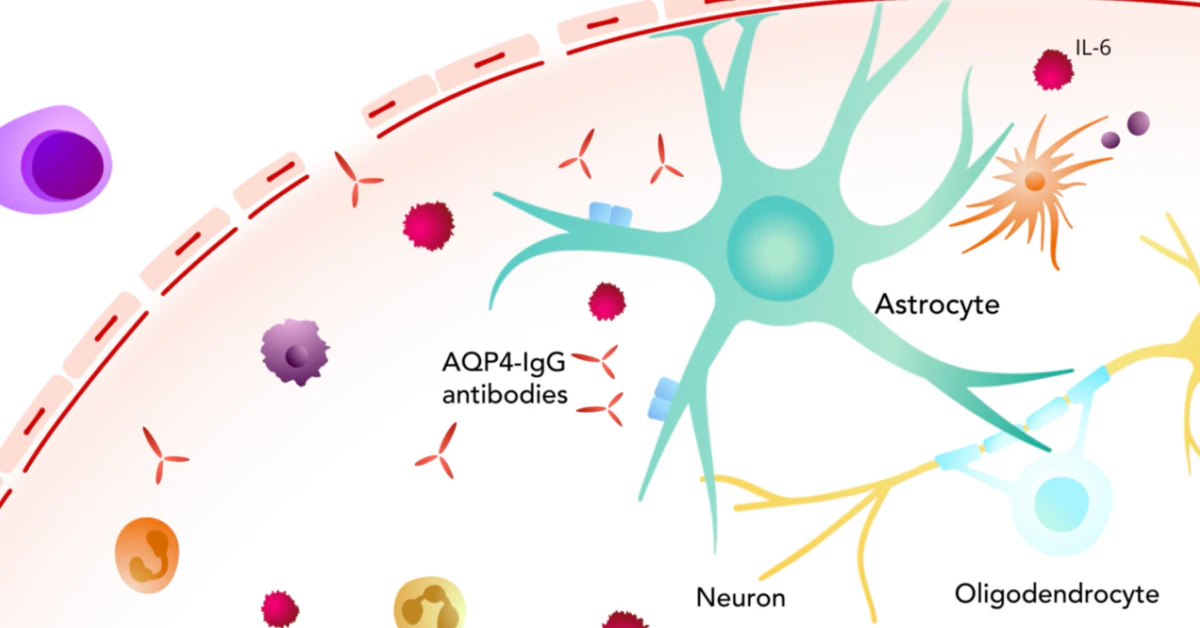
Why IL‑6 Matters
IL‑6 is like the town crier of the immune system. When a pathogen shows up, IL‑6 shouts, “Mobilise the troops!” That shout triggers three critical steps that, in NMOSD, become dangerous:
- Th17 Cell Growth: IL‑6 pushes naïve T‑cells to become Th17 cells, which are pro‑inflammatory and love to infiltrate the central nervous system.
- Plasmablast Survival: It helps B‑cells mature into plasmablasts that churn out the harmful AQP4‑IgG antibodies.
- Blood‑Brain‑Barrier Breach: IL‑6 loosens the tight junctions of the blood‑brain barrier, allowing those antibodies and immune cells to slip into the brain and spinal cord.
Once AQP4‑IgG reaches astrocytes, the complement cascade is activated, leading to astrocyte injury, demyelination, and the painful relapses NMOSD patients dread. By targeting IL‑6, we intervene right at the source of the chaos.
How ENSPRYNG Binds
Satralizumab is engineered with a “Fab” fragment that latches onto both soluble and membrane‑bound IL‑6 receptors with high affinity. Think of it as a universal key that can lock both the front door and the side door of a house. Its IgG2 backbone is specially tweaked to recycle through the neonatal Fc‑receptor (Fc‑Rn), giving the drug a longer half‑life—so patients don’t need weekly shots.
In simpler terms, the antibody sits on the receptor, blocks IL‑6 from attaching, and stays in circulation long enough to keep the blockade steady.
Interrupting the Cascade
When ENSPRYNG blocks the IL‑6 receptor, several downstream signals stop firing:
- STAT3 Phosphorylation—the molecular switch that turns on inflammatory genes—drops dramatically.
- Th17 Differentiation slows, meaning fewer aggressive T‑cells.
- Plasmablast Production wanes, so the pool of AQP4‑IgG‑making cells shrinks.
- BBB Permeability improves, keeping harmful antibodies out of the CNS.
The net result? Fewer relapses, less tissue damage, and more days spent doing the things you love instead of worrying about your next attack.
Clinical Proof That It Works
Two pivotal Phase 3 trials—SAkuraStar (monotherapy) and SAkuraSky (in combination with immunosuppressants)—showed that patients receiving ENSPRYNG experienced roughly a 50 % reduction in the risk of relapse compared with placebo. The benefit emerged within the first 12 weeks and persisted through the four‑year extension studies.
| Feature | ENSPRYNG (Satralizumab) | Tocilizumab | Sarilumab |
|---|---|---|---|
| Target | IL‑6R (soluble + membrane) | IL‑6R | IL‑6R |
| Dosing route | Subcutaneous pre‑filled syringe | IV / SC | Subcutaneous |
| Half‑life | ~28 days (Fc‑Rn recycling) | ~13 days | ~21 days |
| FDA approval for NMOSD | 2020 | — | — |
These numbers are not just statistics; they translate into real‑world stories of patients who go months—sometimes years—without a flare. One participant told us, “I could finally take a weekend trip without packing a hospital bag.”
Benefits You’ll Feel
Beyond the hard data, the mechanism of action brings several tangible perks:
- Reduced Relapse Frequency: Most users report a marked drop in flare‑ups, which means fewer hospital visits and less time on high‑dose steroids.
- Convenient Subcutaneous Injection: The therapy can be self‑administered at home after a brief training session—no infusion centre needed.
- Long‑Term Safety Signals: In the trials, the most common side effects were mild—nasopharyngitis, headache, and injection‑site irritation—while serious infections remained low when patients were screened appropriately.
If you’ve ever felt the anxiety of waiting for the next relapse, imagine swapping that dread for a predictable, steady rhythm. The science behind it—a precise blockade of IL‑6 signaling—makes that possible.
Risks and Monitoring
As with any immune‑modulating drug, there are trade‑offs. Blocking IL‑6 can blunt the body’s fever response and make certain infections harder to spot. Here’s what you should keep an eye on:
- Infection Risk: Nasopharyngitis and cellulitis were the most frequent; rare cases of serious bacterial infections have been reported. According to FDA safety data, patients should be screened for active infections before each dose.
- Hepatitis B Reactivation: The drug can coax dormant hepatitis B virus back to life. Baseline HBV testing is mandatory.
- Liver Enzyme Elevations: Periodic liver function tests are standard practice.
- Neutropenia: A drop in neutrophil count can increase bacterial risk; CBC monitoring every three months is advised.
| Monitoring Item | Frequency | Why It Matters |
|---|---|---|
| TB & Hepatitis B Screening | Before initiation, then as clinically indicated | Prevent reactivation of latent infections |
| Liver Enzymes (ALT/AST) | Every 12 weeks | Detect early hepatotoxicity |
| Complete Blood Count | Every 12 weeks | Watch for neutropenia |
| Infection Surveillance | Continuously—report any fever, cough, or skin changes | Catch infections before they become severe |
Balancing benefits against these risks is a conversation you’ll have with your neurologist. The key is proactive monitoring—think of it as checking the weather before heading out.
Getting Started with ENSPRYNG
Here’s the typical dosing schedule, straight from the prescribing information:
- Loading Phase: 120 mg subcutaneously every two weeks for the first three injections (weeks 0, 2, 4).
- Maintenance Phase: 120 mg every four weeks thereafter.
Administer the injection into the abdomen or thigh, rotating sites each time. Keep the syringe upright, clean the skin with an alcohol swab, and press the plunger gently. Most patients report only a brief pinch.
For a step‑by‑step visual guide, see our Enspryng injection guide. If you ever feel unsure, your nurse can give you a quick refresher before you go home.
Cost, Insurance, and Access
Enspryng isn’t cheap—U.S. pricing hovers around $13,000 per 120 mg dose. That adds up, especially with monthly maintenance. However, several avenues can ease the financial burden:
- Patient Assistance Programs: Genentech’s foundation offers co‑pay relief and, for qualifying patients, free medication.
- Insurance Prior Authorization: Most commercial plans cover ENSPRYNG once the specialist submits the clinical justification (relapse history, AQP4‑IgG positivity, etc.).
- Pharmacy Discounts: Some specialty pharmacies negotiate lower wholesale prices.
For a deeper dive into pricing, visit our Enspryng price page. Knowing the numbers ahead of time helps you plan and avoids nasty surprises.
Putting It All Together
Understanding the ENSPRYNG mechanism of action is more than a scientific curiosity; it’s the foundation for making informed decisions about your NMOSD journey. By blocking IL‑6 signaling, ENSPRYNG quiets the immune chatter that drives relapses, giving you a steadier, more predictable life. The benefits—fewer attacks, convenient at‑home dosing, and a solid safety track record—must be weighed against infection risk, liver monitoring, and cost. With diligent surveillance and open dialogue with your care team, most patients find the balance tips heavily toward the upside.
What’s your biggest question about ENSPRYNG? Have you started the therapy and want to share how the first weeks felt? I’d love to hear your story—feel free to reach out. Remember, knowledge is power, and together we can turn the tide against NMOSD.




















Leave a Reply
You must be logged in to post a comment.