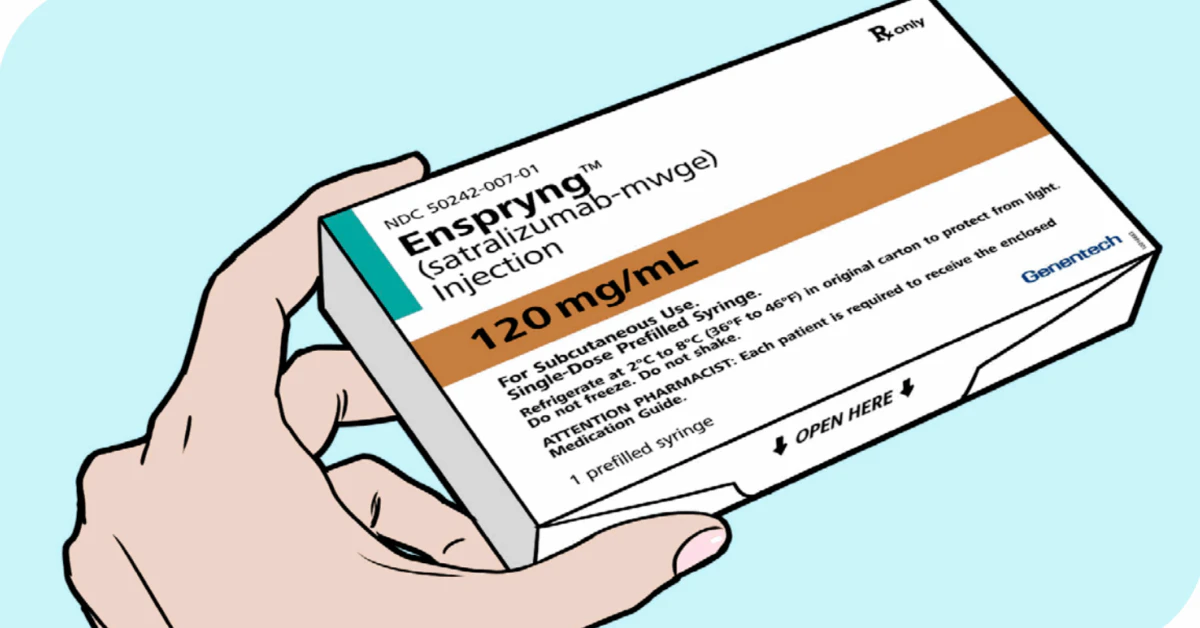
Enspryng ® (satralizumab) is an IL‑6 receptor antagonist that’s been approved by the FDA to help adults living with neuromyelitis optica spectrum disorder (NMOSD) who test positive for the AQP‑4 antibody. Below you’ll find the most useful facts you need before you or a loved one start this treatment – dosage, safety checks, what to expect, and even a quick look at the cost. Think of this as the friendly cheat‑sheet you’d pass to a friend who’s navigating a new prescription.
Quick Look Summary
Key indication: NMOSD in AQP‑4‑positive adults.
Form: 120 mg/mL pre‑filled syringe for sub‑cutaneous injection.
Loading schedule: 120 mg at weeks 0, 2, 4 then 120 mg every 4 weeks (maintenance).
Contra‑indications: Known hypersensitivity to satralizumab, active hepatitis B infection, active or untreated latent tuberculosis.
Top safety concerns: Infections, elevated liver enzymes, neutropenia.
For a deeper dive on the drug itself, check out our Enspryng overview.
Indications & Who?
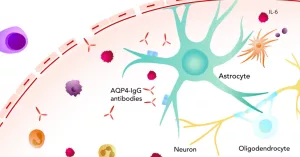
Enspryng is specifically approved for adult patients (≥ 18 years) with NMOSD who are AQP‑4‑antibody positive. The antibody is a hallmark of the disease, and targeting it helps shrink the risk of relapses. In the European Union, the label even extends to patients 12 years and older, but the U.S. FDA has not yet added that age group.
If you’re wondering whether you or a family member might qualify, the first step is a blood test for the AQP‑4‑IgG antibody. A positive result opens the door to this targeted therapy. For those who test negative, other treatments are available, and a neurologist can help weigh the options.
Dosage & Administration
Loading & Maintenance (Featured‑Snippet Answer)
Enspryng is administered sub‑cutaneously at a dose of 120 mg at weeks 0, 2, and 4 (the loading phase), followed by a maintenance dose of 120 mg every 4 weeks.
How to Give the Injection
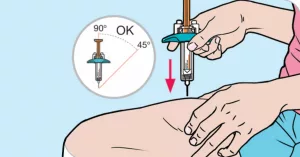
Injecting yourself can feel a bit like learning to ride a bike – a little awkward at first, but once you get the rhythm, it’s smooth sailing. Here’s a step‑by‑step guide you can print out and keep by your fridge:
- Wash your hands thoroughly and gather the pre‑filled syringe, an alcohol swab, and a sharps container.
- Inspect the syringe – it should be clear with no particles.
- Choose an injection site on the abdomen (away from the belly button) or the thigh. Rotate sites each time to avoid irritation.
- Clean the skin with the alcohol swab and let it air‑dry.
- Pinch the skin gently, insert the needle at a 45‑degree angle, and press the plunger completely.
- Dispose of the needle safely in the sharps container.
Need more detail or a visual walkthrough? Our Enspryng injection guide walks you through each step with handy pictures.
Missed Dose & Adjustments
Life happens – travel, illness, or a forgotten reminder. If you miss a dose, take it as soon as you remember, provided it’s less than 14 days late. If it’s been longer, skip the missed dose and resume the regular 4‑week schedule. Never double‑up; staying on schedule is more important than trying to “catch up.”
Dosage Schedule Table
| Week | Dosage (mg) | Notes |
|---|---|---|
| 0 | 120 | Loading dose – first injection |
| 2 | 120 | Second loading dose |
| 4 | 120 | Final loading dose |
| 8 | 120 | Start of maintenance (every 4 weeks) |
| 12 | 120 | Maintenance |
| … | 120 | Continue every 4 weeks |
Mechanism of Action
Ever wonder how a single injection can calm down an immune system that’s gone rogue? The answer lies in the drug’s name – satralizumab – a monoclonal antibody that blocks the interleukin‑6 (IL‑6) receptor. IL‑6 is a cytokine that fuels the production of AQP‑4‑targeting antibodies. By plugging that receptor, ENSPRYNG reduces the inflammatory cascade, giving nerves a chance to heal and preventing new attacks.
For a deeper, science‑savvy dive, see our enspryng mechanism of action article.
Safety Profile & Contra‑indications
Major Contra‑indications
- Known hypersensitivity to satralizumab or any inactive ingredient.
- Active hepatitis B infection.
- Active or untreated latent tuberculosis.
Warnings & Precautions
Infections: Because ENSPRYNG tempers the immune response, any active infection (even a mild cold) should be treated before the next dose. Live vaccines are off‑limits while you’re on therapy. According to the FDA label, “vaccination with live or live‑attenuated vaccines is not recommended during treatment”¹.
Liver enzymes: ALT and AST should be checked before the first dose, then periodically. If levels climb, your doctor may pause treatment.
Neutropenia: A drop in neutrophil counts can increase infection risk. Regular CBCs help catch this early.
Common Side Effects (≥ 15 % incidence)
| Side Effect | Frequency |
|---|---|
| Nasopharyngitis | ≈ 20 % |
| Headache | ≈ 18 % |
| Upper respiratory tract infection | ≈ 16 % |
| Gastritis | ≈ 15 % |
| Rash | ≈ 15 % |
| Arthralgia | ≈ 15 % |
| Fatigue | ≈ 15 % |
| Nausea | ≈ 15 % |
Most of these are mild and tend to improve after the first few weeks. If something feels “off” or you notice a rash that spreads quickly, give your neurologist a call right away.
Patient Counseling & Medication Guide
Before the first injection, you’ll go through a screening package that includes hepatitis B testing, a TB skin test or interferon‑γ release assay, and baseline liver enzymes. This may sound like a lot, but think of it as the “pre‑flight checklist” that ensures a smooth journey.
During treatment, keep an eye on these red‑flag symptoms:
- Fever, chills, or persistent cough – could be an infection.
- Yellowing of the skin or eyes – possible liver issue.
- Unusual bruising or bleeding – could signal low blood counts.
- Severe, spreading rash or hives – possible allergic reaction.
When you notice any of these, contact your healthcare team promptly. Most providers will ask you to pause the dose and run a few lab tests before restarting.
Pricing, Insurance & Access
Cost is a practical concern for anyone on a chronic therapy. The wholesale acquisition cost for one 120 mg prefilled syringe hovers around $5,800 in the United States (price can vary by pharmacy and region). Over a year, that adds up to roughly $30,000‑$35,000, which is why many patients explore assistance programs.
Genentech offers several patient‑support options, including co‑pay assistance, the Genentech Patient Foundation (which can provide free medication for eligible patients), and a network of specialty pharmacies that can help with prior‑authorizations.
Looking for an up‑to‑date breakdown of out‑of‑pocket estimates? Our Enspryng price page consolidates the latest data and links to application forms for financial assistance.
Putting It All Together
Enspryng represents a powerful tool in the fight against NMOSD relapses. By blocking IL‑6, it tampers down the immune fire that attacks the optic nerve and spinal cord. The loading‑then‑maintenance schedule is straightforward, and the injection can be done at home after a short training session.
However, the benefits come with responsibilities: pre‑treatment screenings, regular lab monitoring, and vigilance for infections. If you’re comfortable with these steps, ENSPRYNG can dramatically reduce the frequency of painful attacks and give you more “good days” to enjoy.
Remember, you’re not alone on this journey. Your neurologist, pharmacist, and the supportive community at Genentech are all there to answer questions, troubleshoot side effects, and help with insurance hurdles. If you’ve already started ENSPRYNG, how’s the experience so far? If you’re still contemplating, what concerns keep you up at night? Feel free to reach out to a trusted specialist – the right partnership can turn a complex prescription into a hopeful pathway.
Conclusion
In summary, ENSPRYNG ® (satralizumab) is an FDA‑approved IL‑6 receptor antagonist for AQP‑4‑positive NMOSD adults. The regimen of three loading doses followed by monthly maintenance is simple, but success hinges on proper screening, steady monitoring, and awareness of infection risk. While the price tag can be steep, patient‑assistance programs often bridge the gap, making the therapy accessible for many.
Take this guide as a friendly roadmap: check your antibody status, talk to your doctor about the screening steps, mark your calendar for the injection schedule, and stay proactive about labs and side‑effects. With the right information and a supportive care team, you can confidently move forward and focus on what matters most – living your life beyond NMOSD.
















Leave a Reply
You must be logged in to post a comment.