Carvykti (ciltacabtagene autoleucel) is a breakthrough CAR‑T cell therapy approved for adults whose multiple myeloma has come back (relapsed) or stopped responding (refractory) to other treatments. In plain language, it’s a specially engineered version of your own immune cells that hunt down and destroy the cancer hiding in your bone marrow.
If you’ve landed on this page, you’re probably looking for a straightforward answer that cuts through the medical jargon. Think of this as a friendly chat over coffee—I’ll explain how Carvykti works, who can receive it, what benefits and risks to expect, and how it stacks up against similar options. Let’s dive in and demystify this life‑changing therapy together.
How Carvykti Works
At its core, Carvykti is a BCMA‑directed CAR‑T cell therapy. Here’s the story in everyday language:
- Step 1 – Collect your T cells. A short procedure called leukapheresis pulls out a handful of your white blood cells (the “soldiers” of your immune system). It takes about three to six hours, and you can go home the same day.
- Step 2 – Re‑engineer the cells. In a certified manufacturing lab, scientists add a tiny piece of DNA that tells those T cells to recognize a protein called BC – B‑cell maturation antigen (BCMA). BCMA is like a neon sign on most multiple myeloma cells, making them easy targets.
- Step 3 – Expand the army. The modified T cells multiply in the lab for 4‑5 weeks until there are enough “super‑troopers” to fight the cancer.
- Step 4 – Prepare your body. A short pre‑infusion regimen of cyclophosphamide and fludarabine clears out some of your existing immune cells, giving the CAR‑T cells room to settle in.
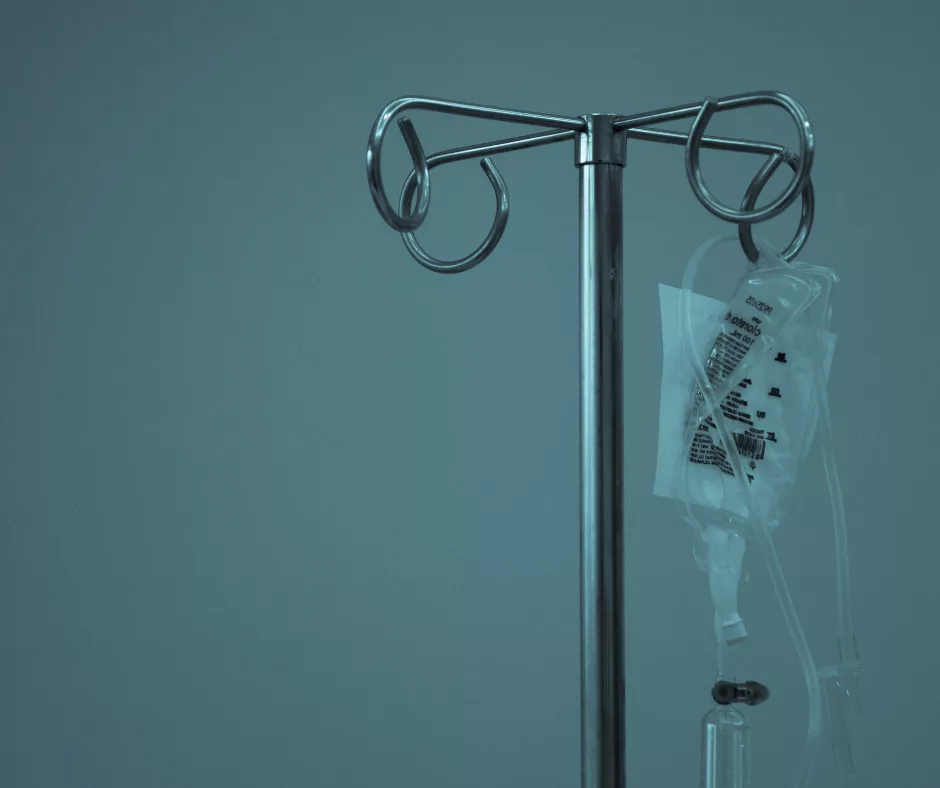
- Step 5 – One‑time infusion. The engineered cells are infused back into you through a vein. From that moment, they seek out and destroy BCMA‑positive myeloma cells, multiplying as they go.
According to the FDA label, the therapy’s mechanism hinges on its dual‑domain BCMA‑targeting design, which gives it high avidity for the cancer cells — a key reason it has shown strong response rates in clinical trials (FDA).
Who Can Get Carvykti
Carvykti is approved in the United States and Europe for adults with relapsed or refractory multiple myeloma (RR‑MM) who have already tried at least one line of therapy that includes a proteasome inhibitor, an immunomodulatory agent, and an anti‑CD38 monoclonal antibody (or are refractory to lenalidomide). In short, it’s for patients who have exhausted the usual options.
Key eligibility criteria include:
- Age ≥ 18 years and able to give informed consent.
- Performance status ECOG 0‑1 (meaning you’re fully active or restricted in physically strenuous activity but ambulatory).
- Adequate organ function (liver, kidneys, heart) as defined by your treating center.
- No active central nervous system involvement or uncontrolled infections.
Special populations—such as patients who have previously received another CAR‑T product, have a history of Parkinson’s disease, or have active CNS disease—require individualized assessment. Your oncologist will weigh these factors carefully before moving forward.
What to Expect During Treatment
Let’s walk through a typical timeline so you know what each phase feels like.
| Phase | What Happens | Typical Duration |
|---|---|---|
| Leukapheresis | Blood is drawn, T cells are filtered out, rest of blood returned. | 3‑6 hours (single day) |
| Manufacturing | Genetic modification, expansion, quality checks. | 4‑5 weeks |
| Bridging Therapy (if needed) | Temporary meds to keep disease in check. | Varies |
| Lymphodepletion | Cyclophosphamide + fludarabine given daily. | 3 days (‑3 to ‑1 before infusion) |
| Infusion | One‑time IV infusion of Carvykti. | 30‑60 minutes |
| Post‑infusion Monitoring | Hospital stay for 7‑10 days to watch for CRS/ICANS. | 1‑2 weeks |
| Long‑term Follow‑up | Regular labs and visits. | Every 3 months for years |
During the infusion, you’ll sit comfortably in a chair; the nurse will attach an IV line and the medication will flow slowly. Most patients report that the actual infusion feels like a routine blood draw—no pain, just a little rustle of the line.
Benefits – Why Carvykti Is Exciting
Clinical data paints a hopeful picture. In the pivotal CARTITUDE‑4 study, 76 % of patients who received Carvykti were alive without disease progression at 12 months, compared with 49 % in the standard‑therapy arm. The overall response rate—meaning the proportion of patients whose cancer shrank dramatically—was over 90 %.
Beyond numbers, many patients experience a genuine return to everyday activities. One 58‑year‑old woman told her oncologist, “I went from being too tired to lift a grocery bag to gardening again in three months.” Stories like that remind us that Carvykti isn’t just a lab‑crafted drug; it’s a bridge back to life.
Risks & Side‑Effects – The Other Side of the Coin
Every powerful therapy carries risks, and Carvykti is no exception. Understanding them helps you and your care team act quickly.
- Cytokine Release Syndrome (CRS). Think of it as an “immune over‑reactivity” that can cause fever, chills, low blood pressure, and rapid heart rate. Grades 1‑2 are usually manageable with supportive care; higher grades may need tocilizumab or steroids.
- Immune Effector Cell‑Associated Neurotoxicity (ICANS). Symptoms range from mild confusion or headache to severe seizures. Early detection is critical—any change in speech, orientation, or coordination should be reported immediately.
- Blood‑cell Suppression. Low neutrophils, platelets, or red cells can increase infection risk and cause fatigue. Regular lab checks help catch problems early.
- Other Common AEs. Fever, nausea, headache, and mild breathing difficulties are typical in the first week.
- Rare but Serious. Very rarely, a secondary malignancy or tumor lysis syndrome can occur. Ongoing monitoring mitigates those risks.
Hospitals that administer Carvykti are required to have a “CAR‑T center” certification, meaning they have 24‑hour ICU support, neurologists, and pharmacists on standby. This safety net dramatically reduces the chance of severe outcomes.
Carvykti vs. Other BCMA‑Targeted Options
When you hear “CAR‑T” you might also hear “Abecma” or “bispecific antibodies” like teclistamab. How do they compare?
| Product | Target | Administration | Key Trial ORR | Approval Year |
|---|---|---|---|---|
| Carvykti | BCMA (dual‑domain) | One‑time IV infusion | ~91 % (CARTITUDE‑4) | 2022 |
| Abecma | BCMA (single‑domain) | One‑time IV infusion | ~84 % (KARMMA‑3) | 2021 |
| Teclistamab | BCMA (bispecific) | Weekly sub‑Q injection | ~63 % (MajesTEC‑1) | 2022 |
If you want a deeper dive, check our Abecma vs Carvykti comparison, which walks through efficacy, safety, and logistical differences.
Cost, Insurance & Access
Financial toxicity is a real concern. The list price for a single Carvykti infusion hovers around $ 450,000‑$ 500,000 in the United States. However, most commercial insurers, Medicare Part B, and many private payers cover it under specialty‑drug benefits—often after prior‑authorization.
Patient‑assistance programs from the manufacturer can offset out‑of‑pocket costs. The “MyCARVYKTI® Patient Support Program” helps navigate insurance paperwork, arrange financial counseling, and sometimes provides co‑pay relief.
For the most up‑to‑date pricing breakdown, see our carvykti price article. It also lists tips for talking to your financial navigator early in the treatment plan.
Real‑World Experience – Voices from the Journey
“When my oncologist first mentioned CAR‑T, I was terrified. The idea of a ‘living drug’ sounded like sci‑fi. After the infusion, I felt a wave of nausea, but the team was there every step. Three months later, scans showed no detectable disease. I’m back to fishing with my grandson now.” – James, 62, RR‑MM survivor.
Dr. Elena Morales, hematology‑oncology specialist at a leading cancer center, adds: “We consider Carvykti when a patient’s disease is aggressive and they have a good performance status. The benefit‑risk balance is compelling, especially given the durability we’re seeing in real‑world data.”
Putting It All Together
Carvykti is a powerful, engineered immune therapy that can turn the tide for many battling relapsed or refractory multiple myeloma. It offers impressive response rates and the possibility of long‑term remission, yet it demands careful monitoring for serious side effects and thoughtful financial planning.
If you or a loved one are facing a multiple myeloma diagnosis, ask your doctor:
- Am I a candidate for Carvykti based on my prior treatments and overall health?
- What will the treatment timeline look like for me?
- How will my insurance handle the cost, and what assistance is available?
- What warning signs should I watch for after the infusion?
These conversations empower you to make an informed decision that aligns with your values and goals. Remember, you’re not alone—there’s a community of patients, caregivers, and experts walking this path together.
Curious about any specific detail? Feel free to explore more about Carvykti on our dedicated page carvykti or reach out to your treatment center for personalized guidance.
















Leave a Reply
You must be logged in to post a comment.