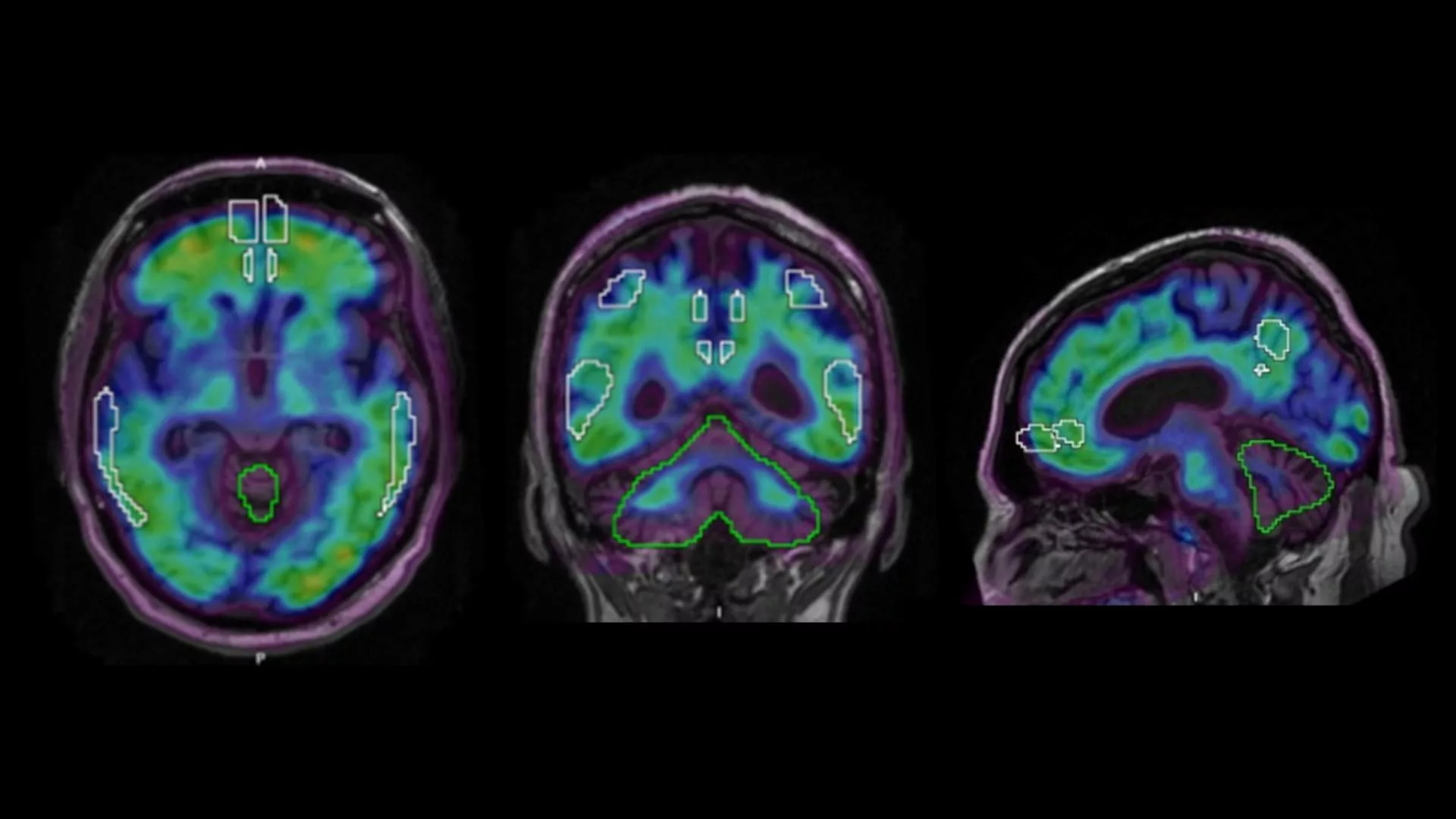
Ever wondered how doctors can actually “see” inflammation buzzing inside your brain? The answer is PET imaging – a molecular‑level scan that lights up the hotspots where glial cells are on high alert. In plain English, it’s a non‑invasive window that shows you what’s happening at the cellular level, helping clinicians diagnose, track, or even guide treatment for a host of neurological conditions.
In the next few minutes I’ll walk you through why PET is considered the gold standard for brain inflammation, what the newest tracers are, how to read the numbers on the scan, and what this all means for patients like you. Grab a coffee, settle in, and let’s explore this fascinating technology together.
Why PET Is Gold
What does “neuroinflammation” actually mean?
Neuroinflammation isn’t just a buzzword; it’s the brain’s immune response when something goes wrong. Microglia – the brain’s resident immune cells – become activated, release cytokines, and can either protect or damage surrounding neurons. This process is a key player in Alzheimer’s, Parkinson’s, multiple sclerosis, and even depression. In short, when the brain gets inflamed, it often signals that disease is either starting or progressing.
How does PET differ from MRI or CT for spotting inflammation?
| Feature | PET | MRI | CT |
|---|---|---|---|
| What it measures | Molecular activity (tracer binding) | Structural anatomy & water content | Bone & dense tissue |
| Resolution | ~4‑5 mm (functional) | ~1 mm (anatomical) | ~1 mm (anatomical) |
| Radiation | Low‑dose (≈ 5 mSv) | None | Higher dose (≈ 7 mSv) |
| Specificity for inflammation | High (tracer‑targeted) | Indirect (e.g., edema) | Very low |
In practice, MRI may look perfectly normal while PET reveals a simmering fire of microglial activation that would otherwise stay hidden.
Which clinical scenarios call for a PET brain‑inflammation scan?
Doctors typically order a PET scan when:
- Symptoms suggest early dementia but MRI is inconclusive.
- They need to monitor how an anti‑inflammatory drug is working.
- Patients are being considered for a clinical trial that requires a baseline inflammation level.
Curious about how this looks in real life? Check out this neuroinflammation PET scan article for a patient‑focused overview.
Safety and the benefit‑risk balance
Radiation exposure from PET is comparable to a standard CT scan and far lower than many other nuclear‑medicine procedures. The tracers themselves are cleared from the body within a few hours, and the procedure is generally well‑tolerated. For most patients, the diagnostic payoff outweighs the modest risk – especially when the scan can prevent a misdiagnosis or guide a life‑changing therapy.
Science of Tracers
Which PET tracers actually bind to inflammatory targets?
The first generation of inflammation tracers targeted the translocator protein (TSPO). Classic examples include 11C‑PK11195 and newer fluorine‑18 versions like 18F‑DPA‑714. While they reliably light up activated microglia, TSPO has a genetic polymorphism that can skew the signal in up to a third of the population.
Enter COX‑2 tracers. A fresh player, 11C‑MC1, binds to the cyclo‑oxygenase‑2 enzyme – a protein that spikes during acute neuroinflammation. A first‑in‑human study involving 27 healthy volunteers showed robust cortical binding and, crucially, dramatically lower between‑subject variability thanks to a surface‑based analysis that respects each person’s unique brain geometry (a study).
How reliable is TSPO as a biomarker?
TSPO remains valuable, especially for chronic conditions where microglial activation is widespread. However, its reliability can be compromised by:
- Genetic differences (the rs6971 polymorphism).
- Low signal‑to‑noise in cortical regions.
- Potential off‑target binding to peripheral immune cells.
Because of these limitations, researchers are increasingly pairing TSPO scans with COX‑2 or other emerging tracers to get a fuller picture.
Why are COX‑2 tracers a game‑changer?
COX‑2 rises sharply in response to acute insults – think infection, trauma, or a flare‑up of neurodegeneration. The newer 11C‑MC1 tracer not only crosses the blood–brain barrier efficiently but also shows a clear distinction between healthy and inflamed tissue. This makes it a promising tool for early‑stage diagnosis and for assessing rapid treatment responses.
How are new tracers validated?
Validation follows a rigorous pipeline:
- Pre‑clinical testing – rodents injected with lipopolysaccharide (LPS) to induce inflammation, plus transgenic mice that overexpress human COX‑2.
- Dosimetry studies – calculate radiation dose per organ in healthy volunteers.
- Kinetic modeling – using two‑tissue compartment models or Logan plots to derive distribution volume (VT) and binding potential (BPND).
- Test‑retest reliability – repeat scans on the same person within a week to ensure consistency.
Where can you learn more?
For an in‑depth dive on tracer chemistry, check out the PET tracer brain inflammation guide.
Reading the Scan
What is the Standard Uptake Value (SUV) and why should you care?
The SUV is a simple, unit‑free number that reflects how much tracer accumulates in a region of interest (ROI) compared with the injected dose normalized to body weight. Roughly:
- TSPO SUV ≈ 1.0–1.5 in healthy cortex, > 2.0 in inflamed areas.
- COX‑2 SUV ≈ 0.8–1.2 normally, climbing above 1.5 when inflammation spikes.
While SUV is easy to compute, it can be influenced by blood flow and timing, so clinicians often confirm findings with more sophisticated metrics.
Understanding VT and BPND
VT (distribution volume) estimates the total amount of tracer in tissue relative to plasma, while BPND (binding potential non‑displaceable) isolates the specific binding component. In the COX‑2 study, surface‑based analysis reduced the coefficient of variation for VT from ~30 % to under 15 %, meaning the numbers are far more reliable across subjects.
Can a single scan tell you the disease stage?
Not entirely, but patterns are informative:
- Alzheimer’s disease – elevated TSPO in hippocampus & posterior cingulate early; COX‑2 may rise during rapid cognitive decline.
- Parkinson’s disease – microglial activation in the substantia nigra and basal ganglia.
- Multiple sclerosis – focal hotspots matching active lesions on MRI.
In practice, the PET results are combined with neuropsychological testing, blood biomarkers, and imaging to stage the disease more accurately.
False‑positives and how to avoid them
A scan can be “hot” for reasons unrelated to neurodegeneration:
- Recent viral infection or vaccination.
- Use of steroids or NSAIDs that modulate TSPO expression.
- High peripheral inflammation bleeding into the brain’s vascular system.
Doctors typically ask about recent illnesses and medications before scheduling the scan, and may repeat the study after a wash‑out period if needed.
Integrating PET with other clinical data
Think of PET as a piece of a puzzle. The typical workflow looks like this:
- Patient presents with cognitive or motor symptoms.
- Baseline MRI and blood work are performed.
- If results are ambiguous, a PET scan is ordered.
- Neurologist reviews SUV/BP values alongside clinical scores.
- Treatment plan (e.g., anti‑inflammatory drug, disease‑modifying therapy) is refined.
This integrative approach boosts diagnostic confidence and helps personalize treatment.
Real‑World Applications
Diagnosing Alzheimer’s versus other dementias
The anatomical specificity of PET inflammation markers is crucial because dementia‑causing diseases affect distinct brain regions. For example, a patient with memory loss and an MRI that shows mild atrophy might still have high TSPO uptake in the entorhinal cortex, pointing toward early Alzheimer’s rather than frontotemporal dementia, which lights up the frontal lobes.
Monitoring response to anti‑inflammatory therapies
Think of the psoriasis study that used FDG‑PET to quantify vascular inflammation after biologic treatment. A similar concept is now being applied to the brain: researchers measure TSPO or COX‑2 binding before and after an experimental drug. A drop in BPND can serve as an early “yes, the drug is hitting its target” signal, even before clinical symptoms improve.
Enriching clinical‑trial participant selection
Trials for disease‑modifying Alzheimer’s drugs now often require a minimum level of neuroinflammation on PET to enroll participants. This “enrichment” strategy reduces variability, increases statistical power, and ultimately speeds up the path to approval.
Future directions – personalized neuro‑inflammation medicine
Experts from a 2020 study envision a future where:
- Hybrid PET/MR scanners provide simultaneous structural and molecular maps.
- Artificial‑intelligence algorithms automatically segment inflamed regions and predict disease trajectory.
- Multi‑tracer protocols (TSPO + COX‑2 + amyloid) give a “four‑panel blood test” in imaging form.
If you’re interested in the broader picture of molecular imaging, have a look at the molecular imaging inflammation post.
Practical Checklist – Getting Ready for Your Scan
Preparation tips
- Fasting: Most centers ask for a 4‑hour fast to stabilize blood glucose.
- Medication review: Bring a list of all drugs, especially steroids, NSAIDs, or recent vaccines.
- Hydration: Drink water before the appointment; it helps clear the tracer after the scan.
What the scanner looks like
The PET scanner is a large, doughnut‑shaped machine. You’ll lie on a comfy table that slides into the ring. The tracer is injected through a small vein in your arm, and you’ll wait a few minutes while it circulates. The scan itself is painless and lasts about 20‑30 minutes – you can even read a short article (like this one) on a tablet while it’s happening.
After the scan
- Results typically arrive within a week.
- Your doctor will explain the numbers in plain language.
- Radiation is cleared quickly, so you can resume normal activities right away.
Cost and insurance
In the United States, a PET brain‑inflammation scan averages between $3,000 and $5,000. Many insurers cover it when it’s medically necessary (e.g., for dementia work‑up). Ask your provider about CPT code 78434 and any pre‑authorization requirements.
Quick FAQs
- Will the tracer stay in my brain forever? No – it decays within a few hours.
- Can I drive after the scan? Yes, the radiation dose is low enough that normal travel is safe.
- Is a PET scan a replacement for a brain biopsy? It’s a powerful, non‑invasive alternative that often provides the needed information without surgery.
Conclusion
PET imaging of brain inflammation has moved from a niche research tool to a practical clinical asset that can illuminate hidden disease processes, guide therapy, and even speed up drug development. Whether you’re a patient navigating a confusing diagnosis, a caregiver seeking clarity, or simply a curious mind, understanding how PET works empowers you to ask the right questions and make informed choices.
If this article sparked any thoughts, feel free to explore the other posts linked throughout – they dive deeper into tracer chemistry, patient stories, and the future of personalized neuro‑medicine. Remember, knowledge is a powerful ally in the journey toward better brain health.


















Leave a Reply
You must be logged in to post a comment.